Adenosine Receptors in Tumors, Thesis Paper Example
Adenosine Receptors in CD73 Tumors as a Diagnostic Marker and Mechanism for Therapy
Abstract
Adenosine triphosphates are the primary source of energy in the transportation system of cells. The breakdown of ATP forms adenosine, a naturally occurring purine. Adenosine is involved in many biological processes. It has been used as an antiarrhythmic agent in heart patients. It plays a role in the immune system by controlling many inflammatory conditions. Adenosine receptors are part of the signaling processes of many different cell types and body systems, such as the circulatory system, the respiratory system, and the nervous system. Adenosine has the capacity to activate pathways in G-coupled receptors and adenosine receptors facilitate the phosphorylation of extracellular signal regulated kinase. Studies have shown that adenosine plays a role as a marker and can be used to study cancer growth using antibody inhibitors. In cancer treatments, adenosine increases cell proliferation while adenosine receptors reduce glioma cell proliferation. Adenosine also is produced during the conversion of AMP and ADP by the enzyme, CD73. CD73 is related to several physiological processes and plays a role in many cancers. It is believed that CD73 can be used as a prognostic biomarker.
Introduction
According to Hulka and Wilcoski (1988), biological markers are “cellular, biochemical or molecular alterations that are measurable in biological media such as human tissues, cells, or fluids” (p. 83). They believe that using biological markers to find treatments or cures of diseases can be an effective method. Hulka and Wilcoski claim that biological markers have the capacity to improve the specificity of exposure variables, to aid in identification of preconditions thus improving chances of prevention, to allow for a more standardized and applicable classification of diseases, and lastly, to enhance researchers’ understanding of the processes that lead to disease occurrence, therefore strengthening researchers questions and interpretations regarding diseases. Mayeux (2004) concurs with Hulka and Wilcoski, but Mayeux has widened the definition to include “biological characteristics that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” (p.182). According to Mayeux, for hundreds of years, biomarkers have been used by epidemiologists, physicians, and researchers in the study of human diseases. Diagnostic markers have aided in the diagnosis and management of heart diseases, nervous system disorders, infections, immunological and chromosomal disorders, and cancers. Biological marker usage has recently increased for two main reasons: a need to have a better way to measure the amount of exposures in the causal pathway of diseases, and a way to gather information about the absorption rates and metabolism of the exposures. Diagnostic biomarkers are of two types: biomarkers of exposure, used in predicting the risk of disease development and also biomarkers of disease which is used in screening, diagnosis, and monitoring the progression of a disease (Mayeux, 2004).
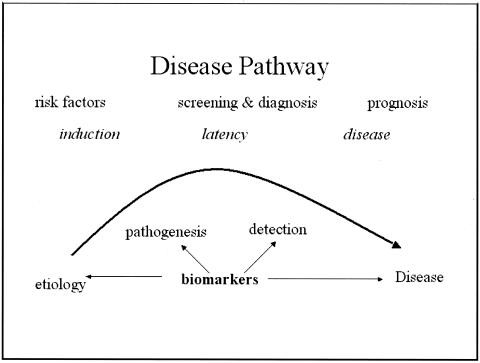
Fig. 1 –Mayeux’s disease pathway and potential impact of biomarkers
Biomarkers have been classified based on the sequence of events from exposure to disease.
A biomarker of disease that has been receiving positive feedback about its capability to identify the causal pathways of many diseases is adenosine. Since the 1929 discovery of adenosine triphosphate (ATP) by Karl Lohmann, a multitude of investigations concerning the nature and properties of adenosine has been conducted (Maruyama, 2003). Adenosine is a purine that is made up of an adenine plus a ribose sugar molecule. It is involved in a variety of biological processes, including the transfer of energy through adenosine triphosphate (ATP). Most ATP is hydrolyzed to adenosine diphosphate (ADP), which can lose another phosphate to become adenosine monophosphate (AMP). Most of the ADP and AMP that form in the cell will have phosphates added in the mitochondria by enzymatic reactions requiring oxygen. If there are large amounts of ATP hydrolyzed, and insufficient oxygen available, then some of the AMP can be further dephosphorylated to adenosine by the cell membrane associated enzyme, 5′-nucleotidase (Klabunde, 2015). One recent research project studied using adenosine, ATP, and their metabolites as biomarkers for cardiovascular protection and also as targets for development of anti-ischemia drugs. Adenosine and ATP are important factors in regulating coronary blood flow, lessening platelet clotting, protecting the myocardium, regulating coronary nerves, regulating the immune system, and energy metabolism (Yeung, 2013).
One of the enzymes that are involved in the process of converting adenosine monophosphate to adenosine is the Cluster of Differentiation 73 (CD73). Michael Arduengo (2013) states that CD73, also referred to as 5’ Ectonucleotidase, is a protein anchored to the membrane that acts at the outer surface of the cell to convert AMP to adenosine and free phosphate. The process of dephosphorization of ATP, ADP, or AMP has an end result of a release of energy within the cell, while the process of phosphating results in storing energy for the cell’s later use. A balance between adenosine and ATP is a necessity for immune homeostasis. ATP is warning signal released by dying or damaged cells that serves to prompt the immune responses and to suppress adenosine production. ATP is then metabolized into ADP, AMP, and adenosine. The conversion of ATP into AMP is catalyzed by the enzyme CD39. Adenosine is then produced by dephosphorylation of AMP. The conversion of AMP into adenosine is reversible by the CD73 once adenosine has become intracellular. CD73 serves as checkpoint between immune activating ATP and immune suppressing adenosine (Shurin, Umansky, & Malyguine, 2013).
Due to the many functions and capabilities, adenosine and its derivatives have been clinically used as signaling agents since the 1940’s (Chen, Eltzschig, & Fredholm, 2013). Previous studies and printed literature on the many uses adenosine its derivatives is why there is strong evidence that adenosine receptors in CD73 tumors can be used as diagnostic markers and as a mechanism for therapy.
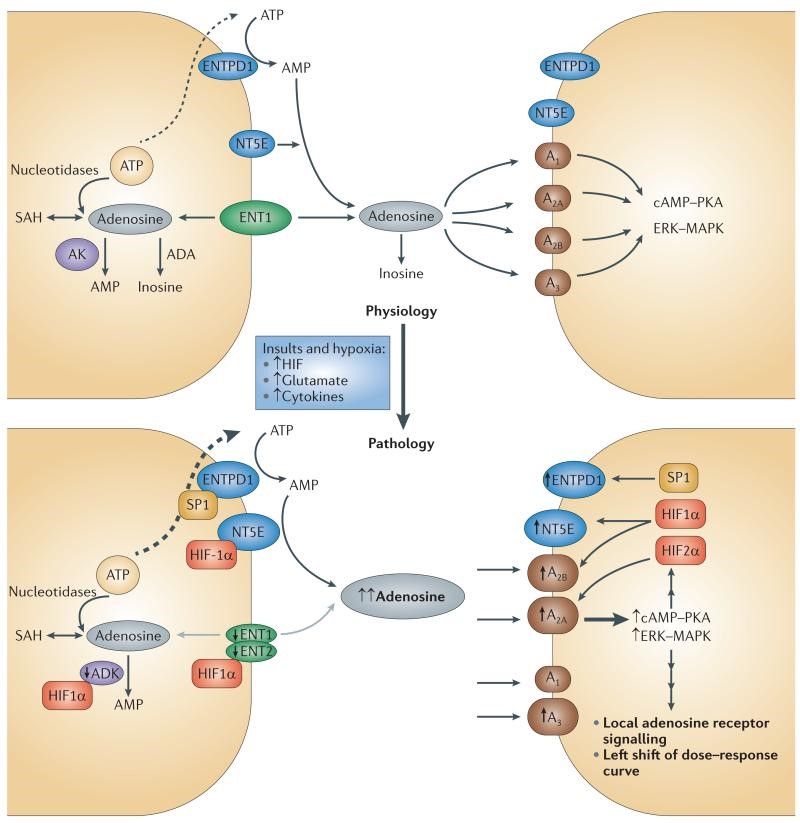
Fig. 2- Local amplification of adenosine signaling in response to insults or hypoxia
Fig 2 –Shows the balancing of adenosine concentration in extracellular fluid. This process includes extracellular adenosine production, adenosine transport, adenosine formation from intracellular adenosine sources and adenosine metabolism to inosine or AMP (Chen, Eltzschig, & Fredholm).
Adenosine Receptors in Different Cell Types. Pelleg & Porter (1990) state that, adenosine is “present in every cell of the human body” (p. 157). Humans have four types of adenosine receptors. Most cells incorporate multiple types of adenosine receptors and their actions may interconnect. Johnston-Cox, Koupenova, & Ravid (2012) categorize adenosine into four types, A1 and A3 as cycle inhibitory receptors and A2a and A2b as adenylyl cyclase stimulatory receptors. A cycle inhibitory receptor exerts inhibitory properties on certain cell functions resulting in cessations of functions or slower functions. An adenylyl cyclase stimulatory receptor exerts stimulatory properties on certain cell functions resulting in switching on functions or increasing speed of those functions (Johnston-Cox, et al., 2012). Adenosine sits at the junction between multiple metabolic and cell signaling cascades. Adenosine can enter and exit the cells by way of nucleoside transporters, thus regulating intracellular and extracellular levels. The normal concentrations of free adenosine in the hippocampus can range from a few nanomolars to hundreds of nanomolars. Quite the opposite, ATP levels can be much higher with the least being 3mM. For this reason, it is more favorable for adenosine to enter neurons by way of the nucleoside transporter. When this fails to happen, adenosine accumulates in the extracellular space (Masino & Boison, 2013).
Adenosine Receptors in Cardiac Cells. Adenosine has regulatory effects on myocardial and coronary circulatory functions. It acts to dilate coronary blood vessels and attenuates beta-adrenergic receptor-mediated intensifications in myocardial contractibility. It also depresses both the sinoatrial and atrioventricular node activities. The effects of adenosine are facilitated by two separate receptors, A1 and A2. A1 adenosine receptors, which are located in the atrial and ventricular myocardium, along with in the sinoatrial/atrioventricular nodes, hold responsibility for the inhibition of adenylyl cyclase activity. A2 adenosine receptors, found in coronary endothelial and smooth muscle cells, are accountable for stimulation of this adenylyl cyclase activity. During periods of greater myocardial oxygen demand caused by rapid pacing and exercise, both coronary blood flow and adenosine concentrations in the myocardium and coronary components also magnified. However, there is no distinct reason explaining this cause and effect relationship at present. The ischemia/reperfusion-induced coronary hyperemia is thought to have been mostly accredited to the released adenosine, therefore it has been confirmed that adenosine lessens the severity of ischemia due to its coronary vasodilatory action. The beneficial effects of adenosine during ischemia/reperfusion is not a clear cut process. The reason why is because myocardial ischemia and reperfusion injury is caused by activated leukocytes and platelets, ATP depletion and calcium overload of myocardium, and catecholamine release from the presynaptic nerves, and the impaired coronary circulation. Interestingly, adenosine weakens all of these unfavorable actions and thus, lessens ischemia/reperfusion injury. Adenosine attenuates the severity of contractile dysfunction (myocardial stunning) and limits the infarct size. For these reasons, administration of adenosine in the ischemic myocardium may be helpful in the reduction of ischemic and reperfusion injuries (Hori & Kitakaze, 1991).
In a study conducted by Albrecht-Kupper, Leineweber, and Nell in 2012, A1 adenosine receptors induced protective and regenerative effects on heart muscles. The benefits of the A1 receptors were conveyed in cardiac muscle cells, the smooth muscle cells, the atria, and the ventricles. According to Albrecht-Kupper and colleagues, when myocardial A1 receptors are activated, they have been proven to inhibit an assortment of symptoms related to ischemia and reperfusion injuries. These pathologies include stunning, arrhythmias, coronary and ventricular malfunctions, acute myocardial infarctions, apoptosis, and chronic heart failures. These inhibitory properties offer new options for A1 receptors in treating conditions like angina, irregular cardiac rhythm, ischemic injuries that occur after acute coronary syndrome, or heart failure.
“Adenosine released during cardiac ischemia exerts a potent, protective effect on the heart” (Liang & Jacobson, 1998, p.6695). Liang and Jacobson state, that the activation of the adenosine receptor, A3 in the cardiac ventricular cell facilitates the protection of ventricular heart cells against injury during subsequent exposure to ischemia.
A study into the protective capabilities of A1 and A3 was conducted in 1997 by Stambaugh, Jacobson, Jiang, and Liang. Stambaugh and colleagues exposed cardiac ventricular myocytes to extended hypoxia and glucose deprivation to recreate ischemia and simulate injuries in chick embryos. When adenosine A3 was present during the episodes of prolonged hypoxia, there was a reduction in the degree of injuries to the cardiac muscle as indicated by a decrease in the amount of creatine kinase released and the percentage of myocytes that died.
The major therapeutic effect of adenosine A1 and A3 is the use of it as an antiarrhythmic agent. Because of its vasodilation properties, adenosine is used in cardiac imaging during stress tests, to determine the severity of coronary flow blockages, and to evaluate pulmonary vasodilatory responses in pulmonary hypertension. Adenosine is administered as a fast acting treatment in cases of supraventricular tachycardia (Klabunde, 2015).
Adenosine is the primary drug used in the treatment of stable narrow-complex SVT, supraventricular Tachycardia. Supraventricular Tachycardia is a rapid heart rate (tachycardia, or a heart rate above 100 beats per minute) that is caused by electrical impulses that start above the heart’s ventricles. Some medical professionals include tachycardia that involve the atrioventricular node, but others do not. Supraventricular tachycardia does not include those tachycardia rhythms that originate from the ventricles (ventricular tachycardia) such as ventricular tachycardia or ventricular fibrillations. Supraventricular tachycardia is also called paroxysmal supraventricular tachycardia and abbreviated either SVT or PSVT (American Heart Association, 2015).
Adenosine is can be given as a rapid IV bolus, because it slows cardiac conduction especially conduction through the AV node. This rapid bolus of adenosine also serves to interrupt reentry SVT pathways through the AV node and restores sinus rhythms in patients with SVT (American Heart Association, 2015).
Adenosine in Cancer Cells. A(1), A(2A), A(2B), and A(3) G-protein coupled cell surface adenosine receptors (ARs) are upregulated in a variety of different cancer cells (Fishman et al., 2009). Activation of certain adenosine receptors may trigger tumor growth. High concentrations of adenosine have been documented in cancer tissues, thereby implicating a relationship between adenosine and growth of tumors (Gessi, Merighi, Sacchetto, & Borea, 2011). Deactivation of certain adenosine receptors shows promise as the basis for a cancer therapy. It seems that receptors, A2A and A3 have the most promising potential as regards to drug development. A2A creates immunosuppressive effects on the tumor, thereby decreasing its anti-tumoral immunity. Safety protocols for this type of treatment has already been tested in Parkinson’s disease (Gessi, et al., 2011).
It is important to gain an understanding of the relationship between adenosine receptor signaling and well known cancer signaling pathways, in order to determine why adenosine receptors are being over expressed in most cancers. Determining why receptors like A(2B)AR are overexpressed, will determine what needs to be done to correct the defect. Adenosine receptor A(2B)AR results in the inhibition of ERK1/2 phosphorylation and MAP kinase activity, which are related to tumor growth signaling pathways. According to Ma, Wang, Duan and Han (2014), ERK1/2 is important to cell survival, proliferation, and differentiation. However, high levels of ERK1/2 has also been connected to tumorigenesis. A study they conducted suggested that ERK1/2 inhibitor will induce production of a protein, IFN-y which is believed to anti-tumorigenic properties (Ma, Wang, Duan, & Han, 2014).
Studies that have analyzed the impact of blocking adenosine receptors in cancer have found that these blocking therapies work by interfering with the growth of cancer cells, but not normal cells. An example of this adenosine receptor inhibition therapy is used in treating melanoma (Fishman, et al., 2001). One study reveals that A(2A)R inhibits T cell functions, therefore, allowing melanoma tumors to develop. Contrary to A(2A)R, A3R has been used to limit melanoma tumor growth in mice by releasing an antitumor immune response (Iannone, Miele, Maiolino, Pinto, & Morello, 2013).
Although there is a characteristic overexpression of certain adenosine receptors in some cancers, it is generally overexpressed in most cancer types. A3 adenosine receptor (A3AR) has enhanced expression in breast cancer and colon cancer (Madi et al., 2004). A3AR has protective properties that are anti-inflammatory, anti-cancerous, and anti-ischemic. This receptor is overexpressed in inflammatory and cancer cells, but is ordinarily expressed in normal cells. For this reason, A3AR has the potential to serve as a therapeutic target (Fishman, Bar-Yehuda, Liang, & Jacobson, 2013).
Adenosine Receptors in the Immune System. Individuals that have defective adenosine deaminase (ADA) will have weakened immune systems. Adenosine deaminase, discovered in 1972 by Dr. Eloise Giblett and colleagues, is found in high levels in the immune’s system thymus. ADA is considered the “housekeeper” of the cell. Its function is to eliminate a molecule called deoxyadenosine. Deoxyadenosine is the end product from the breakdown of DNA. Adenosine deaminase converts the deoxyadenosine, which is toxic to lymphocytes, to a safer molecule called deoxy inosine. Mutations in the ADA gene reduce or eliminate the activity of adenosine deaminase and allow the buildup of harmful deoxyadenosine levels within the cell (Blackburn & Thompson, 2012). This leads to apoptosis in lymphoid cells and these aberrations can manifest as leukemia (Riscoe, Brouns, & Fitchen, 1989).
Adenosine deaminase (ADA) deficiency is a hereditary disorder that impairs the immune system and causes severe combined immunodeficiency (SCID). Individuals with SCID have entirely no immunity from bacteria, viruses, and fungi. They experience repeated and persistent infections that can be extremely serious or life threatening (Immune Deficiency Foundation, 2013). The disease is caused by a mutation in a gene on chromosome 20. This is the gene that codes for the enzyme adenosine deaminase (ADA). Without this enzyme, the body is unable to break down a toxic substance called deoxyadenosine. The toxin accumulates and destroys infection-fighting the immune cells, T and B lymphocytes (Immune Deficiency Foundation, 2013).
In the intrinsic immune system, adenosine is responsible for controlling many inflammatory conditions (from chronic to acute diseases), such as cancer and sepsis. Adenosine deaminase serves as a checkpoint to regulate extracellular adenosine. It monitors adenosine receptors and participates in controlling the activation of stimulation by the adenosine receptors. Thereby, playing a role in regulating purigenic responses to many diseases, such as cancer, chronic lung diseases, rheumatoid arthritis, and sepsis (Antonioli, et al., 2012).
ADA regulates the immune system through adenosine pathways. ADA plays a role in adenosine catabolism. Adenosine catabolism refers to the chemical reactions that result in the breakdown of more complex molecules into simpler forms, such as AMP to adenosine (Aldrich, Blackburn, & Kellems, 2000).
One of the first findings of ADA relationship to the immune system was shown in a study of congenital defects of adenosine deaminase effects on the impairment of T cells and B cells (Aldrich MB et al., 2010). Adenosine deaminase programs a section of the gene shared by the T cell growth factor receptor. Mutations in this gene results in very low T lymphocytes and high numbers of B cells (Immune Deficiency Foundation, 2013). Since then, this kind of research has been of increasing interest to determine how purine metabolism is involved in the immune system.
To regulate the immune system, ADA is helped by macrophages, neutrophils, lymphocytes, and dendritic cells (Antonioli et al., 2012). A study was conducted in 1996 to determine how effective ADA would be on diagnosing tuberculous pleurisy when assisted by neutrophils and T lymphocytes. Burgess, Maritz, Le Roux, and Talijaard (1996) found that the identification of TB in five trials were calculated to be 91%, 81%, 84%, 89%, and 86% using ADA. Adding the lymphocyte/neutrophil ratio to the ADA resulted in increased identification percentages of 88%, 95%, 95%, 88%, and 92%.
Treatment of ADA. Without treatment, this condition is fatal and requires early intervention. Haematopoietic stem cell transplantation is the major treatment for ADA-SCID, even though survival following different donor sources varies greatly. Unlike other SCID forms, 2 other options are available for ADA-SCID: enzyme replacement therapy (ERT) with pegylated bovine ADA, and autologous haematopoietic stem cell gene therapy (GT). Due to the rarity of the condition, the lack of data from many studies, and availability of a variety of treatments, guidance on treatment strategies is limited (Gaspar, et al, 2009).
A consensus management team has reviewed all the literature on this condition and has formulated a theory. The idea is to use matched sibling donor transplants because they represent a successful treatment option with high survival rates and excellent immune recovery. Poorly matched parental donor transplants have a poor survival outcome and should be not be used unless other treatments are unavailable. ERT and GT both show excellent survival, and therefore the choice between ERT, MUD transplant, or GT is difficult and dependent on several factors, including accessibility to the different modalities, response of patients to long-term ERT, and the attitudes of medical professionals and parents to the short- and potential long-term risks associated with different treatments options (Gaspar et al., 2009).
Adenosine Receptors: Signaling Cascades. Adenosine receptors are an important part of the signaling cascade in several different cell types. A1 and A2a receptors are known to work together in the heart to regulate oxygen intake. In addition, adenosine receptor A1 can function as a depressant and reduce heart rate, and the A2A is known to play an anti-inflammatory role (Mustafa et al., 2009). In 1929, the Journal of Physiology published an article concerning the effects of adenosine on the mammalian heart. Their findings revealed that adenosine in the heart slowed the rate of the beating, impaired conduction from auricle to ventricle, and produced auricular fibrillation (Feldman, Cheksis-Feiner, Hamad, & Chan, 2011). In 1963, Dr. Robert M. Berne claimed the adenosine was a critical signaling molecule in the heart. He further hypothesized that adenosine affected the vasomotor tone of the coronary arteries, thereby doubling coronary blood flow (Feldman, Cheksis-Feiner, Hamad, & Chan, 2011).
Mice studies have shown that adenosine receptors have the potential to contribute to the healing process for lung injuries. Healing is promoted because the activation of the A2BAR gene enhances alveolar fluid clearance in the lungs (Eckle, Grenz, Laucher, & Eltzschig, 2008). A study on patients with chronic obstructive pulmonary disease and asthma revealed that “adenosine levels are elevated in the lungs of asthma patients, indicating greater flux through adenosine receptor signaling pathways” (Mohsenin & Blackburn, 2006, p.57).
In the nervous system, adenosine behaves as both a neuromodulator and as a homeostatic modulator. A1R functions by applying a brake on excitatory transmissions (Gomesa et al., 2011). A1R acts directly on nerve cells to inhibit excitability, thus, making it a potent endogenous neuroprotective and anticonvulsant molecule (Masino, Kawamura, Ruskin, Geiger, & Boison, 2012). A2AR works to enhance synaptic plasticity. These receptors have neuroprotective roles (Gomesa et al., 2011).
In the blood, leucocytes and erythrocytes are involved in the adenosine metabolism process. When erythrocytes are not present, adenosine reacts to form inosine and hypoxanthine over time (Heptinstall, Johnson, Glenn, & White, 2005).
CD73. CD73, also known as ecto-5’-nucleotidase, is a glycosyl phosphatidylinositol enzyme located on the cell’s surface that is found in most tissues. Originally, CD73 was thought to be a lymphocyte differentiation antigen that functioned as a co-signaling molecule between T lymphocytes, acting as a binding molecule to attachment lymphocytes to epithelium (Zhang, 2011).
There are two isoforms of CD73 (NT5E) 5′-nucleotidase isoform 1 preproprotein, 574 amino acids; 5′-nucleotidase isoform 2 preproprotein, 524 amino acids. Isoform 2 has the same N- and C-termini but is shorter compared to isoform 1. The NT5E preproprotein is further processed into a mature form, which consists of a dimer of 2 identical 70-kD subunits bound by a glycosyl phosphatidyl inositol linkage at its C-terminus to the external face of the plasma membrane (Zhou & Yang, 2002).
CD73 is a cell surface enzyme found in most tissues and many cell types including lymphocytes, macrophages, dendritic cells, endothelial cells and epithelial cells. Hypoxia induces CD73 mRNA, protein expression and increases CD73 activity in endothelial cells. Particularly, CD73 is highly expressed in many human dense tumors, and its flagrant expression and activity are connected with tumor invasiveness and metastasis and with shorter patient survival. The RNA expression and enzyme activity of CD73 are variable in different breast cancer cell lines (Zhou & Yang, 2002).
Function CD73 is an ectoenzyme (ecto-50-nucleotidase, EC 3.1.3.5). It catalyzes conversion of AMP to adenosine. Adenosine exerts its effects via adenosine receptor A1, adenosine receptor A2A, adenosine receptor A2B and adenosine receptor A3. CD73 has many biological roles, such as regulation of barrier function, adaptation to hypoxia, ischemic preconditioning, anti-inflammation, leukocyte extravasation (Zhou & Yang, 2002).
Expression and activity of CD73 on cancer cells is associated with poor prognosis and may promote metastasis. CD73 accelerates the adhesion, migration, invasion of human breast cancer cells and proliferation of glioma cells and these process are dependent upon the enzyme’s production of adenosine (Zhou & Yang, 2002).
Normal Conditions of CD73. CD73, Cluster of Differentiation 73, plays a role in regulating the duration, magnitude, and chemical nature of adenosine receptor signals delivery to the immune cells. This is accomplished through the conversion of ADP and ATP to AMP followed by AMP to adenosine. After ATP is released into the cells extracellular space, CD39 converts ATP TO AMP, and CD73 dephosphorylates AMP into adenosine (Antonioli, Pacher, Vizi, & Hasko, 2013).
CD73, ecto-5-prime-nucleotidase (5-prime-ribonucleotide phosphohydrolase) catalyzes the conversion at neutral pH of purine 5-prime mononucleotides to nucleosides, the preferred substrate being AMP. The enzyme consists of a dimer of 2 identical 70 kD subunits bound externally to the plasma membrane by a glycosyl phosphatidyl inositol linkage. CD73 is used as a marker of lymphocyte variation. Therefore, a deficiency of NT5E occurs in a variety of immunodeficiency diseases. Other forms of 5-prime nucleotidase exist in the cytoplasm and lysosomes and can be separated from ecto-NT5 by their substrate affinities, necessity for divalent magnesium ion, activation by ATP, and inhibition by inorganic phosphate. It is not known whether the different enzymes are coded by different genes or result from altered posttranslational modifications of a solitary coding sequence (Zhang, 2010).
More recent studies have revealed that CD73 is related to several physiological processes including “epithelial ion and fluid transport, ischemic preconditioning, tissue injury, platelet function, hypoxia, and vascular leak” (Zhang, 2010, p.6407).
Physiological functions of CD73. A primary physiologic function of epithelial cells is water transport. Mucosal tissues lined by epithelia, such as the lung and intestine, achieve this function through a synchronized series of ion transport events. As part of a tissue adaptive response, a number of purine nucleotide metabolites, including adenosine, have been recognized to influence epithelial electrogenic chloride secretion. This is the transport agent in charge of mucosal hydration. This attribute of epithelial function has been studied in detail using models of epithelial cell layers coupled with electrophysiologic strategies. Studies examining the biological characteristics of soluble mediators derived from activated inflammatory cells identified a small, protease-resistant fraction termed neutrophil-derived secretagogue (NDS). It was then developed on epithelia, activated electrogenic chloride secretion and fluid transport. Later biophysical analysis of NDS documented this molecule to be AMP. With no known AMP receptor, studies turned toward defining potential metabolic pathways for adenosine production. Biochemical and pharmacologic studies demonstrated the differentiated expression of CD73 on the surface of cultured and primary intestinal epithelial cells. Further biochemical and morphological studies revealed that CD73 subsists in both a GPI-linked surface fraction as well as in a sub-apical caveolin-rich domain within the epithelium. These expression models have later been shown in a variety of mucosal epithelial cell types (Colgan, Eltzschig, Eckle, & Thompson, 2006).
Barrier properties of CD73. Clearly, CD73 lies central to the regulation of tissue barriers. One study in 2006, proved this when they studied mouse models of intestinal permeability to reveal that oral delivery of the CD73 inhibitor APCP intesifies movement of inert tracers, such as FITC-labelled dextran, across the intestinal epithelium. To investigate changes in vascular permeability in expressed CD73 mice, the researchers used Evan’s blue dye, which binds tightly to plasma albumin. Quantification of formamide-extractable Evan’s blue from individual tissues can then be interpreted as a function of vascular leak. Generally, hypoxia increases vascular permeability two- to four-fold over normoxic conditions, depending on the tissue being observed. Pharmacologic interventions have implied that CD73 is protective under such terms, and most studies have proposed a protective role for adenosine A2 receptors in maintaining barrier function. With all of the evidence it is reasonable to suggest CD73 acts as a gatekeeper for the fine tuning of epithelial and endothelial permeability. Necessary protective pathways can be a common strategy of increasing extracellular adenosine concentrations and promoting adenosine signaling at the cell surface ((Colgan, Eltzschig, Eckle, & Thompson, 2006).
CD73 and Cancer. Many different mechanisms regulate CD73 expression. Understanding these mechanisms could illustrate a clearer functional role of CD73 overexpression in cancer. CD73 has been overexpressed in many different cancer cell lines including, breast cancer, colon cancer, ovarian cancer, stomach cancer, and gallbladder cancer (Gao et al., 2014). Higher expression levels of CD73 has been documented to be associated with tumor neovascularization, invasiveness, and metastasis and also with shorter survival times for patients with breast cancer. Recent studies have revealed that CD73 promotes invasion, migration, and adhesion of human breast cancer cells (Zhang, 2011). CD73 in fact, serves as a signal that contributes to the regulation of the cellular interactions with the extracellular matrix components, including laminin and fibronectin. It is through this signal that helps the cancer to spread (Gao et al., 2014).
CD73 can be used as a prognostic biomarker. Studies in triple negative breast cancer demonstrated that CD73 expression is associated with a worsened prognosis, contributing to predictions of lower survival rates (Gao et al., 2014). Turcotte and colleagues (2014) state that, CD73 is an immunosuppressive enzyme that promotes tumor development and metastasis. The study conducted by Turcotte and colleagues revealed that high levels of CD73 are notably associated with shorter disease free survival and overall survival rate of patients with high grade serious ovarian cancer. Other studies indicate that CD73 has been associated with lymph node metastasis in prostate cancer.
CD73 has both enzymatic and nonenzymatic functions in cells. CD73-generated adenosine plays an important role in the tumor’s ability to escape the immune system. In addition to its enzymatic function, CD73 can also be a signal and adhesive molecule that can modulate cell interaction with extracellular matrix (ECM) components, such as laminin and fibronectin, to mediate cancer invasive and metastatic properties. However, the enzymatic and nonenzymatic functions of CD73 are both involved in the process of caner growth and metastasis, but is not completely independent of each other.
CD73 has been found to be overexpressed in many types of cancers and patient’s biopsies including breast cancer, colorectal cancer, ovarian cancer, gastric cancer, and gallbladder cancer and associated with clinical characteristics, or prognosis of cancer patients. Increasing evidence has verified that CD73 is a key directing molecule in cancer development. In actuality, due to the favorable effect on tumor-bearing mice models, although it has not been investigated in clinical patients, anti-CD73 therapy has become a potential pathway for the treatment of cancer patients in the future (Gao et al., 2014).
A recent study on breast cancer demonstrated that CD73 demonstrated expressed extremely high when associated with the worse prognosis in triple negative breast cancer (n=661, p=0.029 ), but not in patients with luminal (n=2083, p=0.7) or HER2+ (n=487, p=0.86) breast cancer. In contrast, a past study (n=136) reported that positive CD73 expression had a strong correlation with longer disease-free survival (p=0.0044) and overall survival (p=0.027) of breast cancer patients, which suggested that elevated CD73 expression could predict a good prognosis in stages I–III breast cancer patients. Notably, the prognostic implication of CD73 expression in breast cancer remains controversial and is not independent of other clinical studies. A reflective analysis with more clinical specimens will take more factors into consideration, such as patient’s age, subtype, and population, treatment (Gao et al., 2014).
A study was conducted on digestive system cancer to evaluate the clinical significance and prognostic value of CD73 in human gastric cancer (n=68). Analysis of CD73 expression by immune histochemistry (IHC) revealed that CD73 was overexpressed and positively correlated with differentiation of tumor, depth of invasion (p=0.001), nodal status (p=0.003), metastasis (p=0.013), and the cancer stage (p<0.001), and the overall survival rate was low in the patients with high expression of CD73 (p<0.001). In addition to gastric cancer, other studies showed that high levels of CD73 in colorectal cancer (CRC) patients were correlated with a poor prognosis (Gao et al., 2014).
Another study investigated hematologic neoplasm. Investigation of the expression of CD73 in various leukemia subtypes (n=86) revealed that the expression of CD73 was associated with leukemia subtype, differentiation, and development. The clinical implication of CD73 in chronic lymphoblastic leukemia was found to be that high expression of CD73 was associated with behavior that is more aggressive, while another study showed that CD73 expression had no prognostic value in children between ages 1–18 years old with acute lymphoblastic leukemia (Gao et al., 2014).
CD73 in Animal Studies. CD73 has the ability to suppress T cell mediated immune responses by producing extracellular adenosine, which results in overproduction of ATP. However, APCP is an inhibitor that could be used to reduce expression of CD73, thereby promoting tumor regression with the help of B cells and T lymphocytes. Mouse models have been used to demonstrate that inhibiting CD73 would contribute to an improved ability for the immune system to target a tumor through B cell-mediated anti-tumor immunity (Forte et al., 2012). A recent study detected that a CD73 deficiency decreased ovarian tumor development and improved mouse survival in a T cell dependent manner. To receive the best possible effect both the tumor and the host CD73 would have to be inhibited (Wang et al., 2011). In a recent study that used animal models with breast cancer, a monoclonal antibody to CD73 has been shown to reduce the rate of metastasis (Terp et al., 2013). The rate reduction resulted from cell surface clustering of CD73, followed by internalization of the adenosine receptor, thus preventing further signaling that would have else contributed to metastasis (Terp et al., 2013).
Another study has claimed that anti- CD73 antibodies can reduce both primary tumor growth and metastasis of breast cancer. Because CD73 may be expressed in breast tumors that are nonresponsive to current therapies, the antibody could be extremely helpful in patients with advanced disease for whom there are few choices. CD73 is expressed on T lymphocytes and other immune cells. It also plays a role in regulating normal immune responses to inflammation by changing extracellular adenosine monophosphate (AMP) to adenosine. Adenosine lessens the response of immune cells to the site of inflammation, thus dampening immune responses that could ultimately lead to excessive inflammation and tissue damage (Haas, 2010).
CD73 is also expressed in many types of tumor cells. Here its role is producing extracellular adenosine that is thought to help the tumor evade host immunity. In vitro studies have suggested that CD73 can also play a part in breast cancer cell migration. The researchers set out to explain CD73’s role in immune invasion and metastasis of breast cancer as well as to determine whether CD73-targeted therapy could help treat primary and metastatic breast cancer (Haas, 2010).
First, the team demonstrated that murine breast cancer cell tumors are more likely to show metastasis in expressed higher levels of Cd73 and that these cells produced more extracellular adenosine than noninvasive cancer cells. A small molecule CD73 inhibitor decreased adenosine production in cells about to metastasize. Next, they injected immunocompetent and immune compromised mice with the prometastatic breast cancer cells. They then treated the animals with an anti-CD73 monoclonal antibody. The antibody reduced primary tumor growth only in the immunocompetent mice. The team demonstrated that decreased adenosine production allowed immune cells to attack tumors. Contrary to its effect on primary tumors, the antibody reduced the number of lung metastases in both immunocompetent and immune compromised mice compared to no treatment. In vitro examinations showed that the antibody blocked the migration and metastasis of murine breast cancer cells by inhibiting chemotactic signaling between Cd73 and receptor adenosine A2A (Haas, 2010).
Adenosine Signaling Pathway, Membrane Associated Events, and Gene Expression. A signaling pathway is a series of molecular signals that proceeds with an activated receptor promoting the exchange of GDP for GTP on the alpha-subunit of an associated heterotrimeric G-protein complex. The GTP-bound activated alpha-G-protein then detaches from the beta- and gamma-subunits to further transmit the signal within the cell. The pathway starts with receptor-ligand interaction, or for basal GPCR signaling the pathway begins with the receptor activating its G protein in the absence of an agonist, and finishes with regulation of a downstream cellular process, for example transcription. The pathway can start from any structure such as the plasma membrane, Golgi or a nuclear membrane (Klinger, Freissmuth, & Nanoff, 2002).
Adenosine the ability to activate any of the four G-coupled receptors, A(1), A(2A), A(2B), and the A(3) receptors. G protein coupled receptors transfer signals by activating heterotrimeric G proteins. The heterotrimeric proteins interact with the receptor/G proteins and thereby, regulate the signaling reaction. These extra components assist in redirecting the signaling pathway to prompt a specific adenosine receptor (Klinger, Freissmuth, & Nanoff, 2002).
A3 Pathway. Adenosine A3 receptor signaling pathways include activation of G-protein alpha-i family and G-protein alpha-q/11. Adenosine A3 receptors interact with the trimeric G-protein alpha/beta/gamma and stimulate the exchange of GDP to GTP bound to G-protein alpha subunits and the separation of the beta/gamma heterodimers. G-protein alpha-i family inhibits activity of Adenylate cyclase 1, therefore reducing the level of AMP and the activity of Protein kinase in the cell. PKA-cat controls Glycogen synthase kinase 3 beta activity, which is a key component of the Wnt signaling pathway. PKA-cat phosphorylates and inactivates GSK3 beta (Pelleg & Porter, 1990).
Upon activation of Adenosine A3 receptor, non-phosphorylated GSK3 beta phosphorylates and inhibits Catenin, beta 1, 88kDa. Consequently, these events lead to the inhibition of cell cycle progression by decreasing Cyclin D1 – and v-myc myelocytomatosis viral oncogene homolog transcription. G-protein alpha-q/11 activates Phospholipase C beta), which catalyzes hydrolysis of phosphoinositide 4, 5-bisphosphate to form inositol 1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG). The IP3 is released into the cytoplasm and mobilizes Ca (‘2+) from internal stores, whereas DAG activates Protein kinase C epsilon. PKC-epsilon induces PTK2B protein tyrosine kinase 2 beta activation. Pyk2activates V-akt murine thymoma viral oncogene homolog 1 AKT (PKB) through a PI3K -dependent pathway. Pyk2 (FAK2) phosphorylates SHC (Src homology 2 domain containing) transforming protein 1 (Shc) and stimulates protein cascade Growth factor receptor-bound protein 2 (GRB2)/ Son of sevenless homolog (SOS)/ v-Ha-ras Harvey rat sarcoma viral oncogene homolog (H-Ras). H-Ras interacts with the Phosphoinositide-3-kinase, catalytic, gamma polypeptide ( PI3K cat class IB (p110-gamma) ) leading to an increase in its enzymatic activity and catalysis of phosphorylation of PtdIns(4,5)P2 to form phosphoinositide 3,4,5-triphosphate PtdIns(3,4,5)P3 (Pelleg & Porter, 1990).
A signaling pathway initiated by the receptor via G-protein alpha-q/11 and AKT (PKB) activation leads to the stimulation of Conserved helix-loop-helix ubiquitous kinase (IKK-alpha). IKK-alpha phosphorylate Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor ( I-kB ) resulting in dissociation of I-kB from Nuclear factor of kappa light polypeptide gene enhancer in B-cells ( NF-kB ) and NF-kB -dependent transcription. Adenosine A3 receptor survival signaling is coupled with the phosphorylation of cAMP responsive element binding protein 1 (CREB1) through AKT (PKB) -dependent pathway (Pelleg & Porter, 1990).
The Adenosine A3 receptor signaling pathway involves G-protein beta/gamma activation upon it dissociation from G-protein alpha-i family. G-protein beta/gamma activates PI3K cat class IB (p110-gamma) and induces Mitogen-activated protein kinase 1-phosphorylation and Signal transducer and activator of transcription 3 ( STAT3 ) activation via PtdIns(3,4,5)P3/ Ras protein-specific guanine nucleotide-releasing factor 1 ( RASGRF1 )/ v-Ha-ras Harvey rat sarcoma viral oncogene homolog ( H-Ras )/ Mitogen-activated protein kinase kinases 1 and 2 (MEK1(MAP2K1) MEK2(MAP2K2) ) pathway (Pelleg & Porter, 1990).
Stimulation of Adenosine A3 receptor in some cell types results in PI3K- dependent phosphorylation of AKT (PKB) and reduction of basal level phosphorylation of ERK1/2 via v-raf-1 murine leukemia viral oncogene homolog 1 ( c-Raf-1 ) inhibition, which in turn inhibits cell proliferation (Malenka, 2009).
To summarize, the adenosine A (3) receptor has the capabilities to facilitate the phosphorylation of the extracellular signal regulate kinase (ERK1/2), therefore activating this pathway. ERK1/2 operates according to the typical cascade, except that Ca (2+) mobilization is conventional and the novel protein kinase C (PKC) isoforms are not involved in this pathway (Schulte & Fredholm, 2002). The A3 receptor recruits a pathway in which beta/gamma subunits are released from G(i/o), Pl3K, Ras, and MEK to induce ERK1/2 phosphorylation and activation (Schulte & Fredholm, 2002).
A3 Inflammatory Properties. Several other studies have provided evidence to support the theory that activation of A3AR is crucial for anti-inflammatory responses. One study found that, in Rheumatoid arthritis patients, the A3AR agonist CF502 mediated an anti-inflammatory effect by inhibiting the PI3K, PKB/Akt, and nuclear factor-kappaB (NF-?B) signaling pathway. This was demonstrated in murine BV2 microglial cells, activation of A3AR suppresses lipopolysaccharide- (LPS-) induced tumor necrosis factor-? (TNF-?) production through inhibition of PI3-kinase/Akt and NF-?B activation. It was then proposed that selective ligands of A3AR may have therapeutic potential for the management and possible treatment of brain inflammation. In sepsis, A3AR knockout mice had significantly higher levels of plasma TNF-?, increased mRNA encoding cytokines about to be inflamed, and enhanced nuclear translocation of NF-?B in their renal cortices compared with A3AR wild type mice. A3AR WT mice treated with the A3AR agonist IB-MECA showed improvement in renal and hepatic function, showing that A3AR activation presents significant protection from murine septic peritonitis primarily by reducing the hyperacute inflammatory response in sepsis (Ren et al., 2014).
However, the role and mechanism of A3AR in the human colonic epithelial inflammatory response are not clear. Colonic epithelial cells, which act as sentinels of the mucosal immune system, are critical to the barrier and absorptive functions of the colon. Human colonic epithelial cells express numerous inflammatory molecules, including cytokines, chemokines, and receptors, which allow them to communicate with the immune system. NF-?B is critical for the maintenance of epithelial barrier function and modulation of immune responses. The activation of the RelA subunit is a major point in the classical NF-?B signaling pathway, which is required for transactivation of gene expression. NF-?B is sequestered in the cytoplasm of resting cells by inhibitory proteins belonging to the NF-?B inhibitor (I?B) family. Following cell exposure to various stimuli, including LPS and TNF-?, I?B-? is first phosphorylated and rapidly degraded in the proteasomes, allowing NF-?B nuclear translocation and gene activation. Increased activation of NF-?B triggers proinflammatory cytokine expression, resulting in exacerbated colonic inflammatory responses; therefore, suppression of NF-?B signaling may inhibit disease activity in murine models of colitis. It has been reported that adenosine is a negative regulator of NF-?B signaling, resulting in a reduction in interleukin (IL)-8 expression and secretion in human intestinal epithelial cells (Ren et al., 2014).
Membrane Associated Events. In mammals, nucleoside transport is an important structure in plasma and tissue concentration, particularly adenosine. It by this process of transport that adenosine is moved to intracellular space and the immune response is allowed to respond accordingly. Two broad types of adenosine transporters exist, facilitated-diffusion carriers and active processes driven by the transmembrane sodium gradient (Hertz & Matz, 1989). Facilitated diffusion transport is a form of passive transport across a membrane in which a transporter protein facilitates catalyzes the movement of, an otherwise membrane-impermeable molecule or ion, across the plasma membrane down its concentration or electrochemical gradient. Active transport of adenosine is the movement of adenosine across cell membranes and epithelial layers, usually against a concentration gradient, as a direct result of the expenditure of metabolic energy (Hertz & Matz, 1989).
One study by Hertz and Matz in 1989, demonstrated that even though adenosine transport into brain cells is assumed to occur by facilitated diffusion it in fact happens through active transport. The continued net uptake of adenosine depends on its metabolism, which keeps the intracellular concentration of unused adenosine low, thereby maintaining a concentration gradient. As a result, inhibition of adenosine metabolism should decrease uptake. Studies had previously reported a considerable deamination of accumulated adenosine to inosine in primary cultures of cerebral cortical neurons. A moderately detailed adenosine deaminase inhibitor, 2′-deoxycoformycin, was used in the study. Within the presence of that drug, the adenosine content increased without any decrease in total influx of adenosine. Influx of pooled adenosine took place against a concentration gradient, representing that a metabolic degradation of accumulated adenosine is not required to drive adenosine uptake (Hertz & Matz, 1989).
Concentrative nucleoside transporters facilitate the uptake of natural nucleosides and a variety of nucleoside-derived drugs, mostly used in anticancer therapy. The transporters are not distributed evenly in tissues, so therefore is not homogeneous, and their expression can be regulated. In epithelia, CNT and ENT proteins are mostly localized in the apical and basolateral membranes, correspondingly, which results in nucleoside and nucleoside-derived drugs flux. Nucleoside transporters can fulfill roles other than salvages, such as the modulation of extracellular and intracellular adenosine concentrations. Moreover, these transporters also have clinical significance. ENT proteins are target of dipyridamole and dilazep, which is used as vasodilatory drugs in the treatment of heart and vascular diseases. In contrast, nucleoside transporters are responsible for the cellular uptake of currently used anticancer nucleoside-derived drugs, therefore these membrane proteins might play an important role in nucleoside-based chemotherapy. Finally, several polymorphisms have been discovered in CNT and ENT proteins that could affect nucleoside homeostasis, adenosine signaling events or nucleoside-derived drug cytotoxicity (Molina, Casado, & Pastor, 2009).
A1AR and A3AR. The roles of AlAR and A3AR include mediating vasoconstriction. Activation of A1AR increases muscle contraction of vascular smooth muscle, which in turn increases blood pressure. The A1 receptor plays a role in fight or flight situations. (Shepherd, Linden, & Duling, 1996). In vasoconstriction, the A1 receptor interacts with the G-protein trimer and allows for the exchange of GDP to GTP bound to G-protein alpha subunits and the dissociation of the beta/gamma heterodimers. This pathway works in a similar way to the A3R pathway, except that emphasis is on the G-alpha 16->PLC beta1->IP3 and DAG transition (Schulte & Fredholm, 2002).
A study in 2005 was conducted to determine the effects of adenosine on mouse kidneys revealed that adenosine constricted isolated perfused afferent arterioles when added to the mouse’s bath but not when added to the luminal perfusate. Luminal adenosine caused vasoconstriction in the presence of l-NAME or the A2AR antagonist 3,7-dimethyl-1-(2-propynyl)xanthine. The data show that global elevation of renal adenosine causes steady-state vasorelaxation resulting from adenosine 2 receptor (A2AR)-mediated generation of NO. In contrast, selective augmentation of adenosine around afferent arterioles causes persistent vasoconstriction, indicating A1AR dominance. Thus, adenosine is a renal constrictor only when it can interact with afferent arteriolar A1AR without affecting the bulk of renal A2AR at the same time (Hansen et al., 2005).
Although adenosine exerts cardio-and tissue protective effects, the roles and signaling mechanisms of different adenosine receptors in mediating skeletal muscle protection have not been well documented. This study used a mouse hind legs ischemia-reperfusion model to explain the function of three adenosine receptor subtypes. Adenosine A3 receptor-selective agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5?-N-methyluronamide (Cl-IBMECA; 0.07 mg/kg ip) reduced skeletal muscle injury with a significant decrease in both Evans blue dye staining (5.4 ± 2.6%, n = 8 mice vs. vehicle-treated 28 ± 6%, n = 7 mice, P < 0.05) and serum creatine kinase level (1,840 ± 910 U/l, n = 13 vs. vehicle-treated 12,600 ± 3,300 U/l, n = 14, P < 0.05), an effect that was specifically blocked by an A3 receptor antagonist 3-ethyl-5-benzyl-2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS-1191; 0.05 mg/kg). The adenosine A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA; 0.05 mg/kg) also utilized a cytoprotective effect, which was selectively blocked by the A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 0.2 mg/kg). The adenosine A2A receptor agonist 2-p-(2-carboxyethyl)phenethylamino-5?-N-ethylcarboxamidoadenosine (CGS-21680; 0.07 mg/kg)- triggered a decrease in skeletal muscle injury was selectively blocked by the A2A antagonist 2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e] [1,2,4]triazolo[1,5-C]pyrimidin-5-amine (SCH-442416; 0.017 mg/kg). The protection brought by the A3 receptor was canceled in the phospholipase C-?2/?3 null mice, but the protection mediated by the A1 or A2A receptor remained unaffected in these animals. The adenosine A3 receptor is an original cytoprotective receptor that signals selectively via phospholipase C-? and represents a new target for ameliorating skeletal muscle injury (Zheng et al., 2007).
A2A and A2B. The roles of A2A and A2B involves mediating vasodilation. Igor, Feoktistov, and Biaggioni (1996) indicate that A2B receptors have been connected with mast cell activity and asthma, vasodilation, regulating growth of cells, intestinal functions, and regulating nerve secretions.
Adenosine A2B and especially adenosine A2A receptors modulate many physiological and pathological processes in the brain. In this study, evidence relating to the role of such receptors and their potential therapeutic relevance is discussed. The low affinity of A2B receptors for adenosine implicates that they might make a good therapeutic target, since they are activated only under pathological conditions. The availability of selective ligands for A2B receptors would allow examination of such a hypothesis. Since adenosine A2A receptors mediate both potentially neuroprotective and potentially neurotoxic effects, their role in neurodegenerative diseases is highly controversial. Nevertheless, A2A receptor antagonists have shown clear antiparkinsonian effects, and a great interest exists on the role of A2A receptors in Alzheimer’s disease, brain ischaemia, spinal cord injury, drug addiction and other conditions. In order to establish whether such receptors represent a target for CNS diseases, at least two conditions are needed: the full comprehension of A2A-dependent mechanisms and the availability of ligands capable of discriminating among the different receptor populations (Popoli & Pepponi, 2012).
Gene Expression. Genes are composed of DNA that carries information needed to make proteins – the building blocks of our bodies. Variations in the DNA sequence or code of a gene are called mutations, which often are harmless but sometimes can lead to serious disease. Gene therapy treats disease by “repairing” abnormal genes or by providing copies of missing genes.
To reverse disease caused by genetic damage, researchers isolate normal DNA and package it into a vehicle known as a vector, which functions as a molecular delivery truck. Vectors composed of viral DNA sequences have been used effectively in human gene therapy trials. Doctors then infect a target cell, usually from a tissue affected by the disease, such as liver or lung cells, with the vector. The vector delivers its DNA cargo, which then starts producing the proper proteins and restores the cell to normal. However, problems can arise if the DNA is inserted into the wrong place in the genome. For example, in rare instances the DNA may be inserted into a regulatory gene, improperly turning it on or off, leading to cancer (Mandal, 2015).
Researchers continue to improve viral vectors as well as advance non-viral vectors that may have fewer unpredicted side effects. Nonviral gene therapy involves combining DNA with an agent that lets it enter a cell nonspecifically. DNA delivered in this manner is usually expressed for only a small amount of time because it rarely integrates into the host cell genome.
The main efforts of gene therapy is concentrated on delivering a normal copy of a missing or abnormal gene, but current programs are applying gene delivery technology across a broader spectrum of conditions. Researchers are now applying gene therapy in three ways (American Medical Association, 2015).
The first method of gene therapy is used to deliver genes that initiate the destruction of cancer cells or cause cancer cells to regress back to normal tissue. Another technique that is used in gene therapy is to deliver viral bacterial genes as a method of vaccinating the individual against the disease as a preventive method. Researchers are also utilizing a third method of gene therapy. This one involves delivering genes that encourage the growth of new tissue, therefore stimulating regeneration of the injured tissue to repair (American Medical Association, 2015).
Gene Expression Studies. One receptor that is found in all cell types and is generally expressed in very high levels is adenosine. Previous studies have revealed that in adult rats, adenosine A2A gene expression is limited to an area of striatal medium spiny neurons. In a study conducted by Weaver (2003), early developmental gene expression of the receptor A2A is documented in the striatum and parts of the cerebral vasculature. Expressions were also observed in cranial ganglia, carotid body, and intermediate lobe of the pituitary gland.
One study by Christofi et al. (2001) examined adenosine receptors (ADORs) in the enteric nervous system and its importance in the control of motor and secretomotor functions. Gene expression and distribution of neural adenosine A1, A2a, A2b, or A3 receptors (Rs) was studied in the human intestine using immunochemical, Western blotting, RT-PCR, and short-circuit current (Isc). The results were that adenosine A1R, A2aR, A2bR, or A3R mRNAs were differentially expressed in neural and nonneural layers of the jejunum, ileum, colon, and cecum and in HT-29, T-84, T98G, and Bon cell lines. A1R, A2aR, A2bR, and A3R immunoreactivities (IRs) were differentially expressed in PGP 9.5-immunoreactive neurons. A2bR IR occurred exclusively in 50% of submucosal vasoactive intestinal peptide (VIP) neurons (interneurons, secretomotor or motor neurons) in jejunum, but not colon. A2aR was also found in other neurons. A3R IR occurred in 57% of substance P-positive jejunal submucosal neurons (putative intrinsic primary afferent neurons) and less than 10% of VIP neurons. Western blots revealed bands for A3R at 44 kDa, 52 kDa, and 66 kDa. A2aR and A2bR are coexpressed in enteric neurons and epithelial cells. 5?-N-methylcarboxamidoadenosine or carbachol evoked an increase in Isc. A2bR IR is more prominent than A2aR IR in myenteric neurons, nerve fibers, or glia. A1R was expressed in jejunal myenteric neurons and colonic submucosal neurons. Regional differences also existed in smooth muscle expression of ADOR IR(s). It was concluded that neural and nonneural A1, A2a, A2b, and A3Rs may participate in the regulation of neural reflexes in the human gut. Clear cell and regional differences existed in ADOR gene expression, distribution, localization, and coexpression.
Dr. Ananya Mandal (2015) defines gene expression as the process by which genetic instructions are used to synthesize gene products, which are usually proteins that perform essential functions as enzymes, hormones, and receptors. Gene expression is important to the understanding of adenosine signaling because different levels of expressions by the receptor contribute to the modulation of this pathway. However, scientists are currently trying to determine the differential expression patterns that will result from exposing the pathway to different stimuli (St Hilaire, Carroll, Chen, & Ravid, 2009).
Diagnostic Tools and Intervention to Target Adenosine Signaling Cascade. It has been proven that adenosine has a functional role in many diseases. Research into the possibility of targeting adenosine receptors as a diagnostic too has been rapidly gaining support. One example of such research is seen when adenosine is administered at low doses to patients with atrioventricular nodal and atrioventricular nodal reentrant tachycardia. The effect of adenosine on the heart is an abrupt increase in time between beats. This is useful as a diagnostic tool during electrophysiologic studies (Burkart et al., 2002).
Recent Studies. Recent studies have indicated that adenosine deaminase (mediated by RNA dependent adenosine deaminase, ADAR1 which is involved in RNA editing) works as a marker for squamous cell carcinoma of the tongue. Even though little is known about the adenosine signaling pathway in the tongue, it has been shown that significant concentrations of this chemical is detectable when cancer is present Using adenosine deaminase as a marker can act as a minimally invasive technique to help detect the presence of squamous cell carcinoma of the tongue. Furthermore, this detection may have important implications about adenosine signaling that could be used to gain a greater understanding of other cancers (Rai, Kaur, Jacobs, & Anand, 2011).
Other scientist have explored the implications of the presence of adenosine deaminase. Adenosine deaminase has been commonly used as biomarker in the diagnosis of tuberculous pleural effusion. Doctors utilize ADA tests because they are inexpensive, fast, and easy to perform, and provides a preliminary diagnosis before the results of lab cultures are in (Baba, Hoosen, Langeland, & Dyrhol-Riise, 2008). Adenosine deaminase can be assessed using blood cultures and broncho-alveolar lavage fluid. This is a more invasive procedure, but it would likely contribute to a clearer understanding of the relationship between adenosine and the onset and prognosis related to lung cancer (Nikkhoo et al., 2013).
Other studies have shown that the A3 receptor could be targeted for prostate cancer therapy. Fishman et al. (2013) did this when he applied agonists to A3 adenosine receptors in mice models that had been subjected to prostate tumor implants. He then measured protein levels as an indicator of whether there was a receptor expression present. By using the A3AR agonist, Fishman et al. (2013) found that A3AR activation deregulates the Wnt and the NF-kappa B signaling pathways, which helps block the growth of prostate carcinoma cells.
In 2008, Silverman et al. investigated the usefulness of A3 as a biomarker for rheumatoid arthritis. A3 adenosine exerts anti-inflammatory properties and is overexpressed in synovial tissue and peripheral blood cells in patients with active rheumatoid arthritis. This suggests that utilizing A3 adenosine as a diagnostic biomarker for pharmacological abilities and as a therapeutic agent is a promising possibility (Silverman et al., 2008).
Multiple drug resistance has been a well-known problem in human cancer treatments. For this reason, it is important to discover alternative combination therapies that are accessible, as well as to systematically alternate these treatments to make sure that the tumor is not able to develop a resistance to certain types of drugs. For example, in a type of leukemia (AML), nucleoside analogs have been found to be resistant, even though they have components in AML therapy. Plans have been put in place to reverse this resistance by optimizing previously existing nucleoside analogs (Galmarini, Mackey, & Dumontet, 2001).
Many studies have used the adenosine receptor to examine the impacts it has on the growth of cancer using antibody inhibitors. Likewise, there is a significant potential for drugs to be designed in a way that will bind in these same patterns. Currently, the A2A adenosine receptor agonist, regadenoson is the only therapeutic drug that has been approved by the US Food and Drug Administration for this type of cancer (Chen, Eltzschig, & Fredholm, 2013). Regadenoson and adenosine are frequently used vasodilators in myocardial perfusion imaging, which detects obstructive coronary artery disease. In a study to compare regadenoson and adenosine effectiveness, regadenoson produced higher stress MBF than dipyridamole and adenosine: 3.58 ± 0.58 vs. 2.81 ± 0.67 vs. 2.78 ± 0.61 ml/min/g, p = 0.0009 and p = 0.0008 respectively (Vasu et al., 2013).
It is important to consider the process and the lengthy time it takes to create such drugs. Even after pharmaceutical companies find drugs that show potential, it takes approximately 15 to 20 years to conduct clinical trials to submit and evidential application to the FDA for approval. This is why very few effective adenosine inhibitor drugs have been reported in the literature, which is likely due to either the cost of developing these drugs or the lack of trust that these drugs will be successful in diminishing cancer. More research must be conducted to provide evidentiary support for these types of treatments, because currently the only support comes from animal studies and laboratory research.
Many studies aimed at better understanding the adenosine receptor signaling cascade will use inhibitors to determine the levels of protein that result, in order to determine direct and indirect interactions of the steps of the cascade. One method of determining the pathway of receptor signaling cascade is through fluorescent antibodies. Previous studies introduced labelling techniques that utilized fluorescent antibodies into G protein coupled receptors. Further developments of this technique, use fluorescent proteins from Aequoria Victoria to determine localization of isolated cells, organs, and animals. This discovery has assisted in labeling GPCRs and their downstream signaling proteins, in order to be observed by a microscope (Lohse, Nuber, & Hoffmann, 2012). Another commonly used technique to track signaling cascades is the western blots. Western blotting is an important method used in cell and molecular biology. When using a western blot, researchers have the ability to identify individual proteins from complex mixture of proteins removed from the cells. This technique separates by size, transfers to a solid support, and marks the target protein using a primary and secondary antibody visualize (Mahmood & Yang, 2012).
More techniques are needed to conduct such studies because, currently there is a very limited understanding of the interaction between the adenosine receptor and other well-known pathways. However, the relationship between the adenosine receptors and ERK1/2 does indicate that there is an overlap between adenosine receptors and major pathways, but the exact level of impact is not currently known.
Treatment/Therapy Related to Adenosine Signaling and CD73. Even though there has been significant improvements in diagnostic and therapeutic techniques over the past 20 years, many cancers still remain a worldwide public health issue. In particular, triple negative breast cancer (TNBC), with aggressive breast tumors, is associated with a poor prospect for survival, and so far has very few effective therapeutic options. However, recently CD73 has emerged as a potential new target for TNBC in preclinical trials. Also, pharmacological targeting of CD73 and downstream adenosine A2A/A2B receptor signaling is currently an active field of research that could lead to the development of new cancer therapeutics (Allard, Turcotte, & Stagg, 2014).
Anti-CD73 Therapy. Anti-CD73 therapy has become a promising approach for the treatment of cancer patients in the future (Gao, Dong, & Zhang, 2014). A recent study demonstrated that CD73 expression on tumor cells and host cells contribute to tumor development and growth. Yet, when the targeted blockade of CD73 with a monoclonal antibody, the results significantly decreased tumor VEGF levels and suppressed tumor growth in vivo (Allard et al., 2014).
There is promising evidence that targeting CD73 can induce anti-tumor activity in mice. Nevertheless, supplementary experiments are needed before documenting this as absolute fact. First of all, extensive documentation of CD73 expression in a variety of types of human cancers is needed. Secondly, evidence that targeting human CD73 with a therapeutic mAb induces anti-tumor activity is still being decided. Thirdly, in depth examination of anti-CD73 mAb therapy mechanism-of-action is required. Finally, assessment of the possible toxicities that may be associated with CD73 blockade is critical. CD73 is involved in several physiological systems and this could potentially limit anti-CD73 therapy. Past studies in CD73-deficient mice have shown that CD73 is important for platelet aggregation and as a protective mechanism for the heart, kidney and lungs from ischemia. Other studies have identified mutations in the CD73 gene which resulted in a non-functional protein and the development of symptomatic arterial and joint calcification in humans. There is also an increase in ectopic tissue calcification associated with a non-functional CD73 protein, which was found to be dependent on an increase in tissue-nonspecific alkaline phosphatase (TNAP). Anti-CD73 mAb therapy could theoretically be combined with inhibitors of TNAP such as bisphosphonates or lansoprazole in order to prevent the risk of arterial calcification (Stagg, 2012).
While treatment of cancer cells has primarily taken advantage of inhibiting the adenosine receptor, it has been found that APCP treatment contributes to a reduction of 39% in glioma cell proliferation while treatment with adenosine increased cell proliferation by 35%, therefore this inhibitor can potentially help patients that have brain cancer (Gao, Dong, & Zhang, 2014). In a study conducted using mice infected with melanoma, treatment with the CD73 inhibitor, APCP induced a significant tumor reduction. Optimal reduction rates was seen when B cells and T lymphocytes contribute to the process (Li et al., 2014). Clinical studies have not been conducted to determine the usefulness of the APCP inhibitor in all cancers. However, it would be beneficial to test the ability for this chemical to prevent further tumor growth in other cancers that have demonstrated upregulated adenosine receptors. First, it would be beneficial for scientists to complete studies that analyze different cancers to determine which would benefit from adenosine receptor inhibition. Secondly, it is apparent that this technology is promising, even though it is actually under researched.
A very recent study examined combination therapy using the inhibitory role of CD73 on the antitumor efficacy of agonistic anti-CD137 Ab therapy. CD73-deificient mice were treated with anti-CD137 Ab, completely rejected CD73-nonexpressing B16-SIY melanoma growth, when compared with wild-type mice in order to highlight the importance of an inhibitory role for host-derived CD73. To discover the therapeutic relevance of these observations, the researchers combined anti-CD137 and anti-CD73 Ab to treat mice with established B16-SIY melanoma. This combination therapy induced tumor regression associated with enhanced antitumor CD8+ T cell responses and reduced CD4+Foxp3+ regulatory T cells number and function. In contrast, neither anti-41BB nor anti-CD73 monotherapy is effective to control the growth of established melanoma. The study demonstrated that CD73 blockade has critical implications for effective clinical targeting of CD137 and possibly other costimulatory molecules (Chen & Zhang, 2015).
Other Therapies. According to The American Cancer Society (2015), targeted therapy is a newer type of cancer treatment that uses drugs or other substances to more accurately recognize and attack cancer cells. Meanwhile, treatment usually only causes a small amount damage to normal cells. Targeted therapy is a rapidly growing part of many cancer treatment programs. Targeted therapy drugs, like other drugs used to treat cancer, are theoretically considered chemotherapy. But targeted therapy drugs don’t work exactly the same way as regular chemotherapy (chemo) drugs. Take for instance; many targeted drugs seek to damage the cancer cells’ inner mechanisms, the programming that sets them apart from normal, healthy cells. These drugs tend to have different outcome usually with lesser side effects, than standard chemo drugs. Targeted drugs can be used as the primary treatment for some cancers, but in the majority of cases they’re used in cooperation with other treatments such as chemo, surgery, and/or radiation therapy. Most standard chemotherapy (chemo) drugs work by killing cells in the body that grow and divide uncontrollably. Cancer cells divide so quickly, which is the main reason these drugs often work against them. But chemo drugs can also affect noncancerous cells in the body that divide quickly, which can sometimes lead to serious side effects. On top of this, chemo drugs don’t always work against cancer and sometimes they stop working after the body adjusts to them. Targeted therapy drugs work differently. These drugs recognize cancer cells as abnormal and target certain parts of cancer cells that make them different from other cells. However, they may target other cells that help cancer cells grow. Targeted drugs zone in on some of the changes that make the cancer cells abnormal. Because cancer cells can have many different changes in genes, these drugs can attack many different targets. This affects which cancers they might be helpful against, as well as which side effects each drug can cause. Some targeted drugs have the capabilities of being more “targeted” than others. Some might target only a single abnormal protein in cancer cells, while others can go after several different proteins in cancer cells. Some target drugs just boost the way the body fights the cancer cells. Also, this can affect where these drugs work and what side effects they may cause. Targeted drugs are frequently grouped by how they work or what part of the cell they attack.
Target Drugs Types. Monoclonal antibody drugs are an imitated version of large immune system proteins called antibodies. Monoclonal antibody drugs are designed to attack a very specific target on cancer cells and other cells that support cancer. These types of drugs are often referred to as biologics because they are made in cells that are living. The generic names for these drugs, when compared to the brand names, all end in mab. Two examples of this includes, rituximab and panitumumab (American Cancer Society, 2015).
Small-molecule drugs are combined chemicals like most other types of drugs. They are not antibodies. Since antibodies are large molecules, these other drugs are sometimes referred to as small molecule targeted drugs. The generic names for most of these drugs end in ib. Two examples of these drugs include, imatinib and dasatinib (American Cancer Society).
Types of targeted drugs. Targeted drugs can be put into groups by how they work or what part of a cell they target. Several of the more common types used in targeted therapies include, signal transduction inhibitors, angiogenesis inhibitors, apoptosis inducing drugs, immunotherapy drugs, and monoclonal antibodies attached to toxins. Many different targeted drugs are currently being used, and new ones are being developed and released all the time.
Signal transduction inhibitors. The cells in our bodies normally grow and stop growing, in response to chemical signals being picked up from the areas surrounding them. These signals are then transmitted through the proteins to the cell’s control center, the nucleus. In the cell’s center, the nucleus tells it what to do. However, in cancer cells, these signals sometimes get stuck in the on position, and as a result, they tell the cell to grow even without it getting an outside signal. Some targeted drugs block or inhibit proteins that are signals for cancer cells to grow. By blocking these cell signals it can sometimes help keep the cancer under control. However, it’s not clear if any of these drugs alone can cure cancers. Some of these drugs are used by themselves, while others are used along with other treatments, such as chemo (American Cancer Society, 2015).
Examples of signal transduction inhibitors. GFR inhibitors, such as cetuximab (Erbitux) and erlotinib (Tarceva), which are used to treat some lung, colorectal, and other cancer HER2 inhibitors, such as trastuzumab (Herceptin) and pertuzumab (Perjeta), which are used to treat some breast, stomach, and other cancers BCR-ABL inhibitors, such as imatinib (Gleevec) and dasatinib (Sprycel), which are used to treat chronic myelogenous leukemia (CML) and some other cancers ALK inhibitors, such as crizotinib (Xalkori) and ceritinib (Zykadia), which are used to treat some lung cancers. BRAF inhibitors, such as vemurafenib (Zelboraf) and dabrafenib (Tafinlar), which are used to treat some melanomas (American Cancer Society, 2015).
Angiogenesis inhibitors. According to the American Cancer Society, angiogenesis is the process of making new blood vessels. In most cases this is a normal, healthy process, because as the body grows and develops, it needs to make new blood vessels in order to supply blood to all of its cells. Even as adults, there are times when angiogenesis is still important. Take for example, new blood vessels help the body to heal wounds and repair damaged tissue. But this same process can also help tumors grow by providing them their own blood supply. This blood brings nutrients that can allow the cancer to grow and metastasize.
Drugs known as angiogenesis inhibitors prevent tumors from forming new blood vessels, which greatly limits how big they can grow. Many of these drugs work by blocking vascular endothelial growth factor (VEGF) proteins or the VEGF receptors on cells that these proteins use as an attachment point. Unfortunately, these proteins normally help new blood vessels to form around tumors. Some angiogenesis inhibitors only block new blood vessel growth, while several others will affect other targets in cancer cells. Examples of angiogenesis drugs are bevacizumab (Avastin) and ramucirumab (Cyramza) (American Cancer Society, 2015).
Apoptosis-inducing drugs. Several targeted therapies change the proteins inside the cancer cells that cause the cells to deteriorate and ultimately, die. These are called apoptosis-inducing drugs. Apoptosis is another word for cell death. Apoptosis inducing drugs include drugs called proteasome inhibitors, such as bortezomib (Velcade) and carfilzomib (Kyprolis). A proteasome is a complex of enzymes within a cell that helps to destroy proteins that the cell no longer needs. Sometimes in cancer cells, the proteasomes break down proteins that usually would cause the cell to die. This is what allows the cell to keep living. These drugs stop the proteasomes from breaking down these proteins, which in turn makes the cancer cell die (American Cancer Society, 2015).
Immunotherapy drugs. The immune system helps in protecting the body from infections. The immune system can also help protect against cancer, but only to some extent. And, yet it doesn’t do this quite as well. Some drugs help in boosting the immune system’s ability to fight cancer. Several of these drugs behave in a very general way, while others can be considered to be a type of targeted therapy. Examples of immunotherapy drugs include drugs known as immune checkpoint inhibitors.
An important part of the immune system is its ability to keep itself from attacking other normal cells in the body. To do this, it uses checkpoints proteins on immune cells that need to be turned on or turned off to start an immune response. Cancer cells will sometimes find ways to use these checkpoints to avoid being detected and attacked by the immune system. Fortunately newer drugs that target these checkpoints hold a lot of potential as cancer therapies. Examples of checkpoint drugs include drugs that target the PD-1 and PDL-1 proteins, such as pembrolizumab (Keytruda), and drugs that target CTLA-4 protein, such as ipilimumab (Yervoy) (American Cancer Society, 2015).
Monoclonal antibodies attached to toxins. Monoclonal antibodies can be developed to attach to very specific targets on cancer cells. Researchers have now learned how to attach small amounts of a chemical toxin or radioactive substance to some of these antibodies. Once they are inside the body, the antibody serves as a homing device to take the toxic substance directly to the cancer cells, while at the same time not going to other cells in the body. By doing this, it limits the damage done to normal cells (American Cancer Society, 2015).
Immunotherapy. Immunotherapy is a type of cancer treatment that helps your own immune system in fighting cancer. The main functions of the immune system is to help your body fight infections, tissue damage, and other diseases. The immune system is comprised of white blood cells, called leukocytes, organs, and tissues of the lymph system. Immunotherapy is a type of biological therapy. Biological therapy is a type of treatment that uses substances made from living organisms to treat cancer. One of the main reasons that cancer cells is able to thrive is because have the ability to evade and hide from your immune system. Certain immunotherapies can mark cancer cells so it is easier for the immune system to find and destroy them. Other immunotherapies improve your immune system responses in order to work better against cancer (American Cancer Society, 2015).
Types of Immunotherapy. There are many different types of immunotherapy that are used to treat cancer (American Cancer Society, 2015). Monoclonal antibodies, which are drugs that are designed to find and bind to very specific targets in the body. This causes an immune response that results in death of the cancer cells.
Other types of monoclonal antibodies can mark cancer cells so it is easier for the immune system to find and destroy them. When these types of monoclonal antibodies are used it is referred to as targeted therapy.
Adoptive cell transfer is another type of immunotherapy. This is a treatment that attempts to boost the natural ability of your T cells to fight cancer. T cells are a type of white blood cell that develops in the thymus and is part of the immune system. Adoptive cell transfer procedure involves the researchers taking T cells from the tumor. They then isolate the T cells that are the most active against your type of cancer or restructure the genes in them to make them better able to find and destroy your cancer cells. Researchers then grow large batches of these T cells in the lab. Then, treatments to reduce immune cells are administered. After these treatments, the T cells that were grown in the lab will be given back to you through a needle in your vein. The process of growing your T cells in the lab can take 2 to 8 weeks, depending on how fast they grow. Treatment Vaccines, which work against cancer by increasing your immune system’s active response to cancer cells. Treatment vaccines are different from the ones that help prevent disease.
BCG, which stands for Bacillus Calmette-Guérin, is an immunotherapy that is used to treat bladder cancer. This is a weakened version of the bacteria that causes tuberculosis. When inserted directly into the bladder via a catheter BCG causes the immune to respond against the cancer cells. BCG is also being studied in other types of cancer as a potential treatment option.
Cytokines are proteins that are made by the body’s cells. These proteins play crucial roles in the body’s normal immune responses and also in the immune system’s ability to respond to cancer. The two main types of cytokines used to treat cancer are called interferons and interleukins.
Other therapies have been developed in a manner that enhances the effects of other already existing cancer treatments. Another type of cancer treatment involves using cytokines. Cytokines are signaling proteins that are manufactured by white blood cells. The two types used in cancer treatments are interferons (INFs) and interleukins (ILs). Researchers have determined that INFs enhance a patient’s immune system by activating natural killer cells and dendritic cells. ILs increases the proliferation of white blood cells, including cytotoxic T cells and killer cells, while also facilitating the production of B cell antibodies (National Cancer Institute, 2013). In one study, a model of cytotoxic T lymphocyte-associated antigen 4 (CTLA-antibody (mAb) was assessed in combination with either modulators of adenosine receptors (AR) activation or an inhibitor of adenosine production in a murine model of melanoma (Iannone, Miele, Mailolino, Pinto, & Morello, 2014). The study demonstrated that the combination therapy was more beneficial to enhance the effects that are already seen by adenosine receptor inhibitors (Iannone et al., 2014).
Since there are many mechanisms that cause immune suppression in tumor tissue, it is only logical to create therapies that counteract these effects. Combination chemotherapy accomplishes three important objectives not possible with single-agent therapy. First, it provides the maximum cell kill within the range of toxicity level tolerable by the host for each drug. Secondly, it offers a wider selection of coverage of resistant cell lines in a heterogeneous tumor population. Lastly, it prevents or hinders the development of new drug-resistant cell lines (National Cancer Institute, 2013).
Currently, combination chemotherapy is customary treatment for the most advanced cancers. However, evidence supporting the advantages of combination therapy is mixed. In one study of breast cancer, an evaluation of 12 trials, involving 2317 randomized women, compared the effect of combination chemotherapy to the same drugs given sequentially in women with metastatic breast cancer. The researchers concluded that sequential single agent chemotherapy will have a positive effect on progression-free survival, although combination chemotherapy has a higher response rate and a greater risk of febrile neutropenia in metastatic breast cancer. There were no differences in overall survival time between these treatment therapies even when the subgroups were analyzed. In particular, there was no difference in survival according to the schema of chemotherapy (giving chemotherapy on disease progression or after a set number of cycles) or according to the line of chemotherapy (first-line versus second- or third-line). In the researchers supported the recommendations by international guidelines to use sequential monotherapy unless there is rapid disease progression (National Cancer Institute, 2013).
One specific type of therapy that has promising potential in combating immune suppression is using a combination of CD73 or A2A receptors as a counter agent. One study that compared combination therapy versus single agent therapy in metastatic breast cancer revealed that combination therapy increased survival rate by 18% (Miles, Minckwitz, & Seidman, 2002). Gaining a better understanding of signals and other therapies is beneficial in combination therapies because it can be used in a way to help support the initial treatment. There are few therapeutic treatments available for adenosine receptor blocking, therefore it is reasonable to continue investigating the inhibitors that currently exist using this method to ensure that maximum effectiveness is obtained.
Additional studies have shown that anti-CD73 therapy helps contribute to a blocking of angiogenesis. It is important to determine how this system could be used to take advantage of the adenosine receptors interactions to contribute as a medical benefit (Allard et al., 2014). In order for cancer cells to proliferate, they need an abundance of nutrition and oxygen. The high density of angiogenesis is able to support the sustenance required for tumor cells to grow. In addition to nutritional supply, angiogenesis is also the main pathway for cancer cells to invade distant tissues. By infiltrating into immature vessels, tumor cells get carried into distant organs by the blood. Therefore, abundant tumor angiogenesis is required for cancer growth and metastasis (Gao et al., 2014). One study revealed that even though CD73 expression on tumor cells and host cells contribute to tumor angiogenesis, targeted blockage of CD73 is showing promise as a possible treatment. Since tumor derived CD73 will enhance the production of endothelial growth of the tumor target blocking CD73 with a monoclonal antibody significantly reduced VEGF levels and suppressed tumors angiogenesis in vivo (Allard et al., 2014)
It has been found that CD73 can rescue anti-tumor T cells from the immunosuppressive effects of extracellular adenosine. This is important conclusion because rescuing T cells can help support the immune system’s ability to fight cancer. T cells make up a major component of adaptive immunity and aid in fighting against infections and tumors. Adenosine can affect T cells activity by increasing the amount of intracellular AMP levels. Also, adenosine has been found to inhibit IL-2 production in tumors, thus preventing clonal development of immunologically active antitumor T cells. One study reported that overexpression of CD73 on tumor cells encouraged T-cell death in vitro and limited the antitumor effect of T-cell in vivo. However, these impairments could be repaired from decreasing the level of CD73 expression (Gao et al., 2014).
Analyzing the impacts of receptor interaction with other protein levels in the body is helpful to gain an understanding of the biological effects of an anti-CD73 therapy, because understanding these processes will more effectively contribute to trials that will result in a FDA approved therapy.
Discussion
It is not unreasonable to formulate a conclusion that adenosine receptors can be used as a biomarker for CD73 tumors. Adenosine receptors play a role in a variety of physiological functions throughout the body. Adenosine receptors are characterized into four groups, with each separate receptor having its on effect on the body. Adenosine receptor A1 expresses as inhibitory functions in most of the tissues it occupies. Stimulation of adenosine receptor A1 has a depressant effect on the heart. It reduces the speed of electrical pulses conducted, thus lowering the heart rate. It is because of adenosine receptor A1, that adenosine makes a useful medication for individuals with tachycardia. Adenosine receptor A2A works with A1in a protective role for myocardial tissue. Adenosine A1 receptor has a vasodilator functions, thereby works to lower blood pressure. Adenosine 2B receptor works to stimulate adenylate cyclase to start the process of catalyzing Adenosine triphosphate (ATP). Adenosine 2B serves as a guard for the cell, sending out the warning signal when too much adenosine has accumulated in the extracellular fluid of the cell. This receptor is also has vasoconstrictor properties. Adenosine receptor A3 serves as an immune booster by inhibiting the effect of adenosine as a free agent.
When Adenosine monophosphate needs dephosphorylating CD73 is the enzyme that acts as the catalyst to start the process. CD73 also has a function which allows it to promote tumor growth and metastasis. Hypothesizing, since CD73 and adenosine monophosphate have an over lapping relationship, it is not a far stretch to believe that adenosine receptor A3 can be used as a biomarker for CD73 tumors.
Scientists have used adenosine receptors in numerous research studies. The results have shown potential for adenosine receptors to be used in therapeutic programs for cancer and diseases that are immunoevasive. However, it seems that the full potential of adenosine receptors comes when they are paired with another receptor that has a similar effect. One study revealed that adenosine receptors when supported by T lymphocytes and B cells from the immune system, greatly improved the end results from the treatment programs. The use of adenosine receptors with a combination therapy should be an effective treatment plan. If adenosine receptors are used to identify CD73 tumors, it will be even more effective to use monoclonal antibodies attached to toxins to destroy the abnormal tumor cells. The process of diagnosing and identifying tumors should become easier. Scientist and medical professionals will be able to identify a high expression of adenosine receptors at the surrounding tissues and the site of the tumor. There may even be a way to manipulate what receptor needs to express highly and what receptor needs to reduce the amount of expression. Scientist are consistently seeking ways to separate and identify what receptors will work best. Much progress has already been made. Drugs are already developed that work to help the heart and lower and raise blood pressure.
Technology has made many leaps and bounds from just a few years ago. It is not unconceivable to believe that some scientist will discover a way of curing cancer. However, until then treatments and therapies must be effective in fighting cancer. Cancer is a horrific, devastating disease that doesn’t discriminate. For many years chemical chemo was the main treatment for cancer. But along with the cancer cells, normal healthy tissues were destroyed. Patients receiving chemo was fatigued, listless, and despondent with no appetite. A body in a weakened state also has a weakened immune system. An immune system that can’t even fight off a common cold, surely will not be cancer. That is why alternative treatments must be effective.
Investments in both time and dedication as well as financial support must be put forth to support research that has the potential to change millions of lives. Pharmaceutical companies and the Food and Drug Administration must work together to make new medications safe and affordable for everyone.
Society must remain optimistic. Adenosine receptors studies haven’t been conducted for many years. It sometimes takes years and years to make breakthrough discoveries in the fields of medicine and science.
References
Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. (2014). Anti-CD73 Therapy impairs tumor angiogenesis. Int J Cancer. 134(6):1466-73.
Albrecht-Kupper B, Leineweber K, Nell P. (2012). Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal, 8:91-97.
Aldrich MB, Blackburn MR, Kellems RE. (2000). The importance of adenosine deaminase for lymphocyte development and function. Biochem Biophys Res Commun, 272: 311-315.
American Heart Association. (2015), Web. Retrieved from http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/High-Blood-Pressure-or Hypertension_UCM_002020_SubHomePage.jsp
American Cancer Society. (2015). Web. Retrieved from http://www.cancer.org/
Antonioli L, Colucci R, La Motta C, Tuccori, M, Awwad O, Da Settimo F, Blandizzi C, Fornai 1. (2012). Adenosine Deaminase in the Modulation of Immune System and its Potential as a Novel Target for Treatment of Inflammatory Disorders. Current Drug Targets, 13(6): 842
Benke PJ, Dittmar D. (1976). Purine dysfunction in cells from patients with adenosine deaminase deficiency. Pediatr Res, 10: 642-646.
Chen J, Eltzschig HK, Fredholm BB. (2013). Adenosine receptors as drug targets — what are the challenges? Nat Rev Drug Discov., 12(4): 265–286.
Eckle T, Grenz A, Laucher S, Eltzschig HK. (2008). A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest., 118(10):3301-15.
Fishman P, Bar-Yehuda S, Barer F, Madi L, Multani AS, Pathak S. (2001). The A3 adenosine receptor as a new target for cancer therapy and chemoprotection. Exp Cell Res.,269(2):230-6.
Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S, Borea PA. (2009). Adenosine receptors and cancer. Handb Exp Pharmacol., (193):399-441.
Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock AM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. (2012). Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol., 189(5): 2226–2233.
Galmarini CM, Mackey JR, Dumontet C. (2001). Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia, 15(6): 875-890.
Gao Z, Dong K, Zhang H. (2014). The Roles of CD73 in Cancer. BioMed Research International, 2014.
Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. (1972). Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet, 12: 1067-1069.
Gomesa CV, Kastera MP, Toméa AR, Agostinhoa PM, Cunha RA. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1808(5): 1380–1399.
Haas M. (2010). CD73:Double breasted suit. SciBx, 3(4).
Heptinstall S, Johnson A, Glenn JR, White AE. (2005). Adenine nucleotide metabolism in human blood–important roles for leukocytes and erythrocytes. J Thromb Haemost., 3(10):2331-9.
Hertz L, Matz H. (1989). Inhibition of deaminase activity. NeuroChem, 14(8):755-60.
Hirschhorn R. (1977). Adenosine deaminase deficiency and immunodeficiencies. Fed Proc, 36: 2166-2170.
Hulk B.S., Wilcoski T. (1988). Biologic markers in epidemiologic research. Arch Environmental Health. 43(2): 83-89.
Iannone R, Miele L, Mailolino P, Pinto A, Morello S. (2014). Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am J Cancer Res, 4(2):172-181.
Immune Deficiency Foundation. (2015). Immune deficiency foundation handbook. Web. Retrieved from http://primaryimmune.org/idf-publications/patient-family-handbook/
Johnston-Cox H, Koupenova M, Ravid K. (2012). The role of adenosine in response to vascular inflammation. Journal of American Heart Association. Retrieved from doi: 10.1161/ATVBAHA.112.246181
Klabunde, R. (2015). Cardiovascular pharmacology Concepts. Web. Retrieved from http://www.cvpharmacology.com/antiarrhy/adenosine.
Madi L, Ochaion A, Rath-Wolfson L, Bar-Yehuda S, Erlanger A, Ohana G, Harish A, Merimski O, Barer F, Fishman P. (2004). The A3 adenosine receptor is highly expressed in tumor versus normal cells: potential target for tumor growth inhibition. Clin Cancer Res., 10(13):4472-9.
Masino S, Boison D. (2013). Adenosine: A key link between metabolism and brain activity. New York: Springer Science.
Mayeux, R. (2004). Biomarkers: Potential uses and limitations. NueroRx.,1(2):182-188.
Mustafa SJ, Morrison RR, Teng B, Pelleg A. (2009). Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol., (193): 161-88.
National Cancer Institute. (2015). Web. Retrieved from http://www.cancer.gov/
Nikkhoo B, Sigari N, Ghaderi B, Abdolrahim A, Namam-Ali A, Behzad M, Fardin F,
Mohammad A. (2013). Diagnostic Utility of Adenosine Deaminase in Serum and Bronchoalveolar Lavage Fluid for Screening Lung Cancer in Western Iran. Journal of Medical Biochemistry, 32(2): 109-115.
Pelleg, A., Porter, RS. (1990). The pharmacology of adenosine. Pharmacotherapy., 10(3):157-74.
Rai B, Kaur J, Jacobs R, Anand SC. (2011). Adenosine deaminase in saliva as a diagnostic marker of squamous cell carcinoma of tongue. Clin Oral Investig., 15(3):347-9.
Riscoe MK, Brouns MC, Fitchen JH. (1989). Purine metabolism as a target for leukemia chemotherapy. Blood Rev, 3: 162-73.
Schulte G, Fredholm BB. (2002). Signaling pathway from the human adenosine A(3) receptor expressed in Chinese hamster ovary cells to the extracellular signal-regulated kinase 1/2. Mol Pharmacol., 62(5):1137-46.
St Hilaire C, Carroll SH, Chen H, Ravid K. (2009). Mechanisms of induction of adenosine receptor genes and its functional significance. J Cell Physiol.,218(1):35-44.
Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. (2013). Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol., 191(8): 4165-73.
Zhang B. (2010). CD73: A novel target for cancer immunotherapy. Cancer Res., 70(16): 6407–6411.

Time is precious
don’t waste it!

Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee