Clinical Trials for the Remittance of Diabetes Mellitus Type II, Research Paper Example
Background: The American Diabetes Association estimates by the year 2034, there will be 44.1 million individuals living with Diabetes Mellitus Type II in the United States. Estimated cost for annual treatment of this disease by the year 2034 is to be approximately $336 billion. This is triple the current cost to treat those living with the chronic condition at the present time. This form of diabetes is normally due to an individual being overweight or obese. Many times it can also be genetic and be cause simply by the body not producing enough insulin for the amount of glucose produced. Patients with diabetes have no cure, only a regimen of maintenance, which consists of medication and diet modification as well as exercise. Many times this will help keep the disease from progressing and often it will not.
Worldwide, estimates by the year 2030 are 438 million people living with diabetes. Diabetes Mellitus Type II and obesity have been linked and a person’s risk of obesity directly increases their risk of Diabetes Mellitus Type II. Almost 90% of newly diagnosed Diabetes Mellitus Type II patients are overweight or obese. A person with a Body Mass Index (BMI) of 30-35 has double the risk of diabetes development as a healthy person and a person with a BMI of 40+ has 10 times the risk (ADA).
Diabetes Mellitus Type II is known to have several related secondary conditions, among these are vascular conditions such as retinopathy, nephropathy, neuropathy, cardiovascular disease, cerebrovascular disease, and peripheral vascular disease (ADA). Patients with Diabetes Mellitus Type II will often have sores that do not heal or cuts and scrapes that become infected because of the diabetes-related slow healing process. Often times diabetes suffers will lose one or more limbs to amputation because of infection from a simple wound that has gotten out of control. It is known that obesity will increase the morbidity and mortality of patients with Diabetes Mellitus Type II if the conditions are directly linked (Cummings et al). Due to the global increase of Diabetes Mellitus Type II and the increase spending in treatment for the disease and its secondary conditions, cost-effective strategies should be investigated.
This study intervention is based on literature review. Literature has shown conventional treatment for Diabetes Mellitus Type II has limited success and is not sustainable with obese and severely obese patients (ASMBS).
Gastric sleeve surgery aims to reduce weight and maintain weight loss through reducing food intake and modifying the physiological changes that drive an individual to regain weight. This surgery also aims to cause rapid and sustained improvements in glycemic control as well as sustained improvements in micro and macro vascular damage (IDF).
Gastric sleeve surgery is one of the newest forms of bariatric surgery; bariatric surgery has been used to help in obesity cases for many years with a good ratio of success. The gastric sleeve procedure is performed under general anesthesia and the bariatric surgeon removes 85% of the stomach. It then appears in the shape of a tube. The surgeon then closes this tube shaped stomach with staples. Normally, the entire procedure is performed laparoscopically (ASMBS).
There are cases when this particular type of surgery is followed by a traditional gastric bypass surgery or a duodenal switch surgery after the patient has had a significant weight loss. This protocol is known as a ‘staged’ approach, and the gastric sleeve procedure helps decrease the risks of the traditional gastric bypass surgery, which would be followed (ASMBS).
Gastric sleeve surgery is normally performed only when a person has a Body Mass Index of 40 or above; this would equate to the person being about 100 pounds overweight. However, if the person has other secondary problems, such as diabetes or high blood pressure or cholesterol, bariatric surgeons will perform this surgery on a patient with a Body Mass Index of around 35 (depending on the surgeon) (IDF).
According to the Journal of the American Medical Association in 2004, gastric sleeve surgery resulted in a loss of 62 to 75% of patients’ original weight. Individuals with diabetes mellitus type II reported remission in 76.8% of cases and significant improvement with their condition in 86% of cases. Hypertension was eliminated in 61.7% of cases and significantly improved in 78.5% of cases. High cholesterol was reportedly reduced in more than 70% of cases and sleep apnea was 85.7% eliminated (ASMBS).
Research also has shown that metabolic surgical measures such as gastric sleeve surgery may have a significant impact on the improvement of insulin resistance and secretion. This would be completely separate from any weight loss incurred. Researchers believe this improvement could be the result of changes in the gastrointestinal hormones and have reported that many of their patients with Diabetes Mellitus Type II have experienced complete remission within mere days of having metabolic surgery. This obviously would be much sooner than the patient losing a significant amount of weight (ASMBS).
Many physicians believe, also, that because the ‘hunger’ hormones in the stomach are affected when a portion of the stomach is removed, patients are better capable of controlling their appetites and following a more strictly modified diet for a longer period of time than before the surgical procedure was conducted. The ‘hunger hormones’ are still under investigation, but have not been ruled out as a significant source of problems for those slightly to morbidly obese patients with diabetes mellitus type II having problems controlling their weight even with diet, exercise, and medication modifications (ASMBS).
Study Question: Is gastric sleeve surgery more effective in remitting Diabetes Mellitus Type II than conventional treatment (diet, exercise, and medication) among adults (ages 18-60) with DM2 and a Body Mass Index equal or above 35 over a post surgical period of 24 months?
Primary Hypothesis: The use of gastric sleeve surgery is more effective in remitting Diabetes Mellitus Type II than conventional treatments among adults with DM2 and a Body Mass Index equal or above 35 over a period of 24 months after surgery.
Null Hypothesis: The use of gastric sleeve surgery is not effective in the remittance of Diabetes Mellitus Type II when compared to conventional treatments among adults with DM2 and a Body Mass Index equal or above 35 over a period of 24 months after surgery.
Primary Objective: Reduce weight by reducing patient’s Body Mass Index to 25 or less over the 24 months and/or by reducing waist circumferences (men<40 and women <35 inches).
Secondary Objectives: Reduce patient’s blood pressure to <130/80 mm Hg over the next 24 months.
Reduce patient’s blood glucose level for a fasting glucose of <126 mg/dL and HbA1c <7.0%.
Decrease patient’s blood lipids profile. Resulting goals are: total cholesterol <200 mg/dL; triglycerides <150 mg/dL; HDL ? 40 mg/dL (men); HDL ? 50 mg/dL (women); LDL <100 mg/dL (optimal goal); LDL <70 mg/dL (very high risk patients).
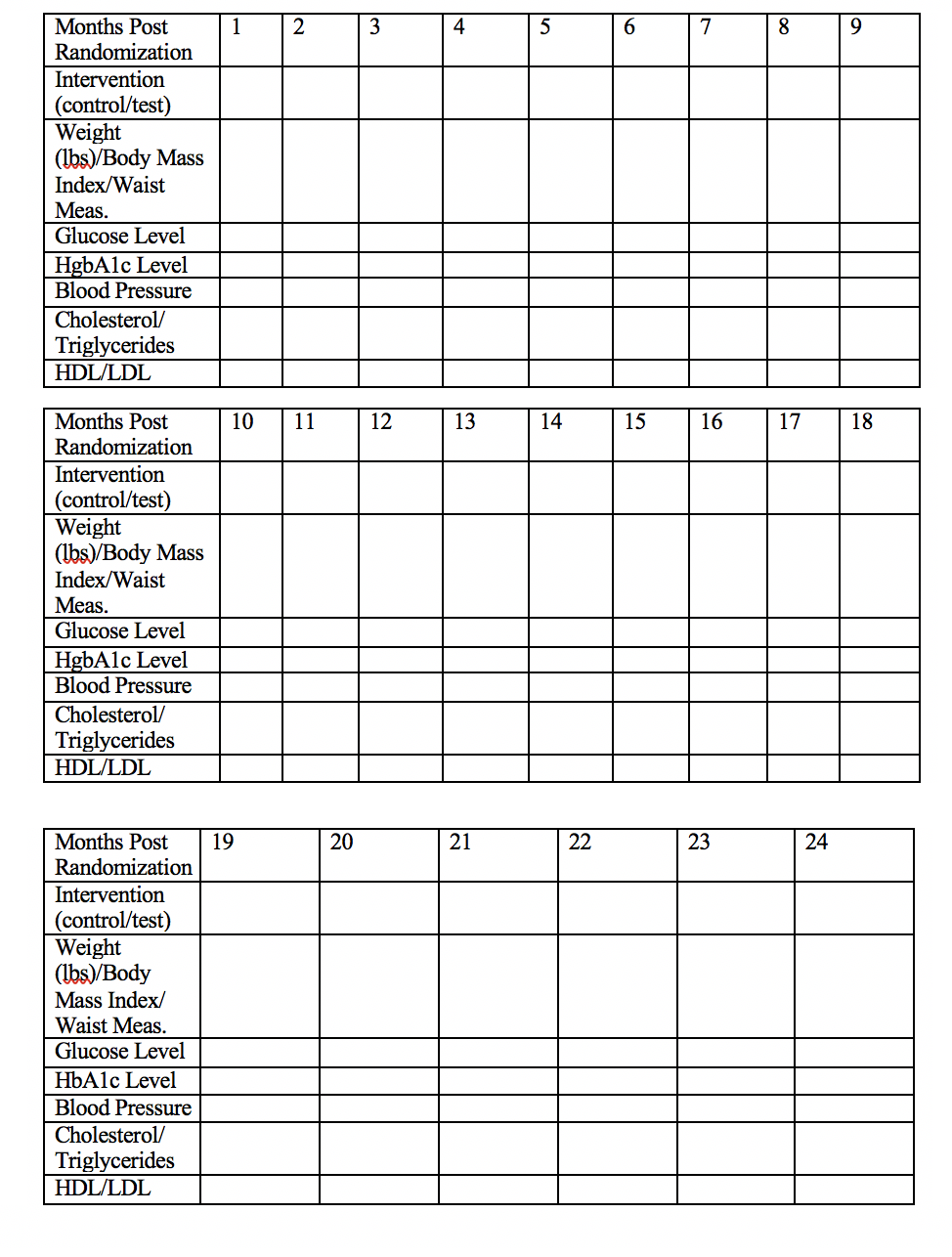
Measurements taken: For the primary objective, patients will be weighed on a standardized scale once per month at the Endocrine clinic. They will also have their waist measurement taken. For secondary objectives, patients will be asked to measure their own blood pressure once per day and will also have blood pressure measured during once per month at the clinic. Patients will also be asked to measure their glucose via a glucose monitor three times per day and an HbA1c level will be taken once per every 3 months at the clinic. To determine blood lipids, a fasting lipid profile will be taken once per month at the clinic.
Trial Design: Clinical Randomized Trial: Patients will be randomly placed into either the test group (which will have the gastric sleeve surgery) or the control group (which will follow a conventional standardized method of diet, exercise, and medication).
Study Population: Adults, ages 18 – 60 years, diagnosed with Diabetes Mellitus Type II and a Body Mass Index >35 who are willing and able to commit to post surgical testing for a period of 24 months after bariatric surgery.
Inclusion Criteria: Patients between the ages of 18 – 60 years of age. Patients diagnosed with Diabetes Mellitus Type II at the Endocrine clinic. Patients with a BMI >35. Patients who can tolerate general anesthesia. Patients willing and able to commit for 24 months post surgical follow up testing for weight, measurements, BMI, glucose levels, blood pressure, and total lipid profile.
Exclusion Criteria: Patients with Diabetes Mellitus Type I. Patients younger than 18 or older than 60 years of age. Patients with a BMI < 35. Patients who are unable to tolerate general anesthesia. Patients unwilling or unable to commit to post surgical follow up for 24 months. Patients who may be or are currently pregnant. Patients who are disabled. Patients with a history of any bariatric interventions. Patients who are currently dependent on medications that cause hyperglycemia.
Primary Outcome: Patients undergoing gastric sleeve surgery will have resultant weight loss as measured by a scale once per month at the clinic.
Secondary Outcomes: Patients undergoing gastric sleeve surgery will have a decrease in glucose levels as measured daily by a self-check glucose monitor and measured by HgA1c level once per three months at the clinic.
Patients undergoing gastric sleeve surgery will have a decrease in overall blood lipid profiles as measured once per month at the clinic.
Patients undergoing gastric sleeve surgery will have a decrease in blood pressure as measured once per day by the patient and upon every visit to the clinic.
In addition to the patient outcomes, gastric sleeve surgery and the remittance of diabetes mellitus type II in patients will have a direct relation with a decreased risk of cardiovascular disease. There will also be a decrease in medical costs associated with the maintenance of treating diabetes mellitus type II patients. Patients treated with gastric sleeve surgery will also have a decreased risk of comorbidity diseases that have an indirect relationship with diabetes mellitus type II.
Study Schema:
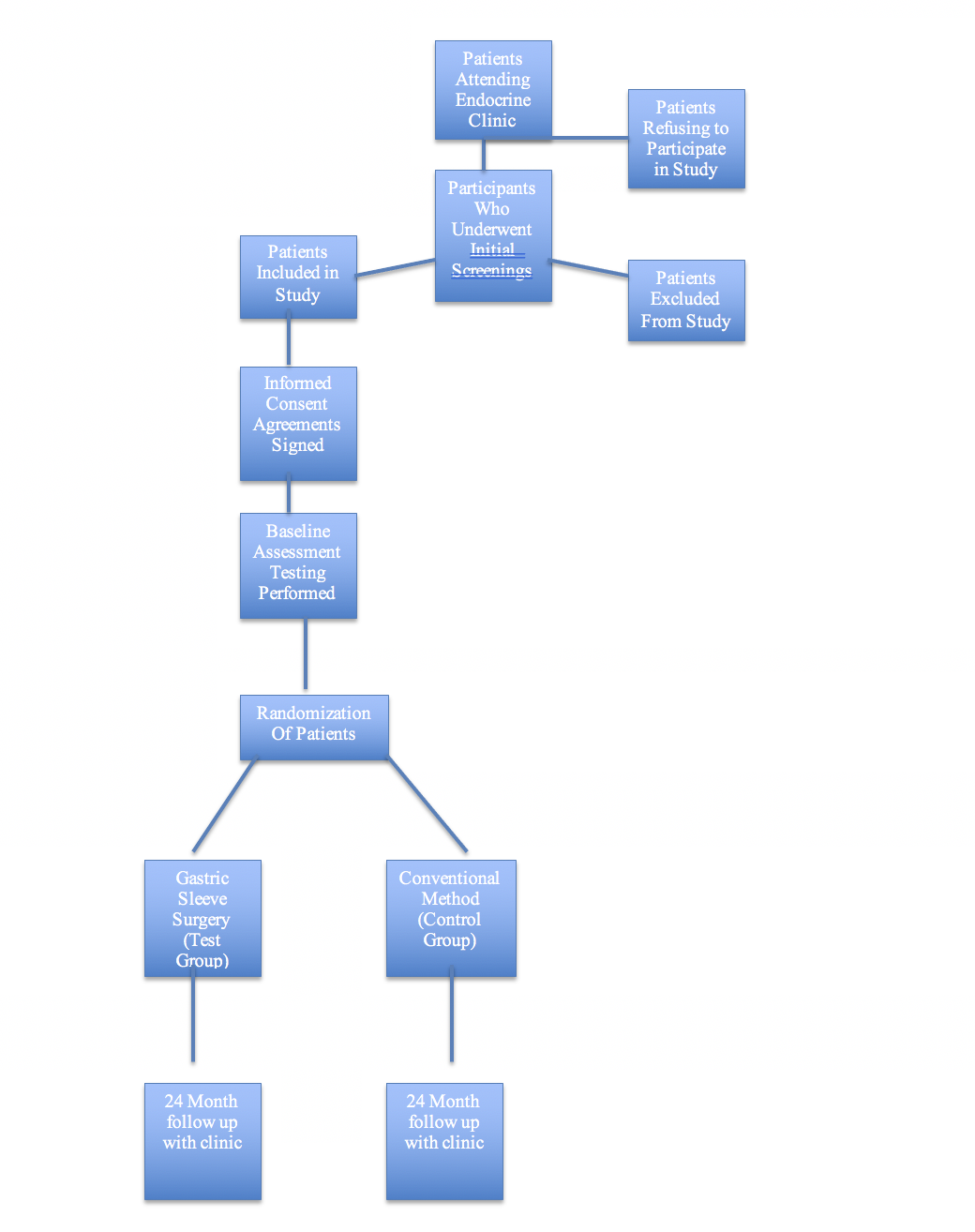
Screenings: All patients visiting the Endocrine clinic will be given the opportunity to participate provided they meet the inclusion criteria. Those patients not meeting the criteria will be classified under the exclusion category.
Informed Consent: All patients participating in the study will sign a statement agreeing to participate in the study and allowing physicians to perform gastric sleeve surgery (if they are part of the test group) as well as agreeing to following all post surgical instructions pertaining to the monitoring of glucose, blood pressure, weight assessment, and returning to the clinic for official follow up every month for 24 months.
IRB Approval. All approval will be obtained through the proper entities with the inclusion of written IRB approval. This approval will be obtained for all aspects of the study, including all assessments post surgical for the duration of the time period, informed consent process and document, procedures and rating instruments, randomization, clinical services provided by the Endocrine clinic as well as all physicians and medical staff administering surgical procedures or assessment to patients or the conventional method group, and any other press releases, videos, or other media related occurrences therein. No advertising or direct soliciting of participants will be initiated without IRB approval of all written flyers, brochures, documents, or other materials.
Baseline Assessment: A baseline assessment will be taken on every patient in the test and control group to include weight, body mass index, waist circumference measurement, blood pressure, glucose, HbA1c, and total lipid profile. This will be used in the statistical analysis during and after the study is completed.
Randomization: Patients meeting inclusion criteria will be randomly assigned a position in the test or control group via a third party method so as to avoid bias. This will take place before any baseline assessments are taken. There should be an equal amount of males and females assigned to each group as well as equal representation amongst age span within the groups.
Gastric sleeve surgery (gastric sleeve surgery): Those patients assigned to the test group will undergo gastric sleeve surgery and begin their postoperative phase testing. This surgery will be performed at a hospital adjacent to the Endocrine clinic by a group of bariatric surgeons who have agreed to commit to follow up for 24 months during our trial.
Conventional treatment (for control group): Those patients assigned to the control group will be provided free meals via a meal delivery service during the entire course of the study so each patient will be consuming the same number of calories, fat grams, carbohydrates, sodium, and other nutritional content. In addition, each patient in the control group will be given a free membership to the hospital fitness center located near the Endocrine clinic during the entire course of the study in order to give all patients the same opportunity for exercise and to give patients the same equipment options as far as weight lifting and cardiovascular methods are concerned. Each patient will continue to take the medication prescribed to him or her by their physician as they begin the ‘post-operative’ phase testing of the study.
Follow up for 24 months: All patients will participate in follow up testing for the duration of 24 months as explained above. The chart below will be used to assess the various categories examined each month for data collection purposes.
Adverse Effects: The monitoring of adverse effects will be important, especially in the first four weeks post surgery for those patients taking part in the gastric sleeve procedure. During the latency between the surgical procedure and the first month’s assessment, patients may report adverse affects to a clinical trial supervisor at the designated telephone number contained in the post surgical materials given to the patient during the day of the procedure. All adverse affects will be explained in the post surgical materials as well as post surgical care for the patient and instructions on patient care until the first assessment one month following the procedure.
After the first month, any adverse affects should be noted upon the scheduled visits for patient follow up at the Endocrine clinic. The same will be true for those patients in the conventional treatment group. Those patients will be given a packet of materials on day one and will be given a number to call with any adverse affects for the first month of the plan. After the first month, any adverse affects should be made known during the follow up visits at the Endocrine clinic.
In the case of any severe adverse effects, please immediately notify the phone number in the patient materials as soon as possible and describe the severe adverse affect so proper instructions may be given as to where treatment can be obtained. This is true for both the patient and test groups. Any severe adverse affects that might occur during the course of the 24 month follow up period are to be immediately reported to the phone number listed so proper treatment may be obtained. All adverse affects will be reported to the proper medical oversight boards in writing no later than three months after the end of the trial.
Analysis of Data: Data analysis of the post surgical follow up testing for those patients in the test group as well as those patients participating in the conventional method group will be computed by using Cronbach’s alpha analysis. With the use of this analysis, all data will be analyzed via separate categories and a statistical change result and ? value will be given for each set of data. This data may then be compared either month to month or control group to test group to assess the rate of success of this clinical trial. All values should be as close to “1” as possible and there will be a two-standard deviation method (95% of all results replicated by any other researchers would be safely assumed to fall within this range). This ensures the utmost validity to the study.
Twenty-Four Month Follow Up Testing for All Participating Patients
Flow Chart of Study Schema
Works Cited
Cummings, J., Kevin Mineo, Richard Levy, and Richard Josephson. “A review of the
DIGAMI study: Intensive insulin therapy during and after myocardial infarctions
in diabetic patients”. Diabetes Spectrum 12.2 (1999): 84-88.
American Diabetes Association (2012). http://www.diabetes.org/
American Society for Metabolic and Bariatric Surgery (2012). http://asmbs.org/benefits-of-bariatric-surgery/
“Bariatric surgical and procedural interventions in the treatment of obese patients with
Type 2 Diabetes”. International Diabetes Federation (2012). http://www.diabetes.org.br/anexo/idf-position-statement-bariatric-surgery.pdf.

Time is precious
don’t waste it!

Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee






