 All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Food & Drug Administration Agency, Research Paper Example
Hire a Writer for Custom Research Paper
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

Introduction
The Food and Drug Administration (FDA) is a department contained within the US Department of Health and Human Services. It has a number of core responsibilities that include: (1) the protection of the public by ensuring the regulation of health and safety measures and provision of security over such items as drugs, vaccines, biological products and other medical products, and (2) providing regulation over tobacco and other items. Another particularly important role of the FDA is fighting against potential pandemic outbreaks and developing new vaccines (US Food and Drug Administration).
Regulating Food
The FDA also has regulatory jurisdiction over food related products that are overseen by other governmental bodies such as the Center for Food Safety and Applied Nutrition (CFSAN). The Center for Food Safety and Applied Nutrition is the governmental body that strives to ensure the food supply is both safe and sanitary, and is correctly labeled. The Table in Fig 1 illlustrates the type of different food related products that fall under the regulatory jurisdiction of the FDA.
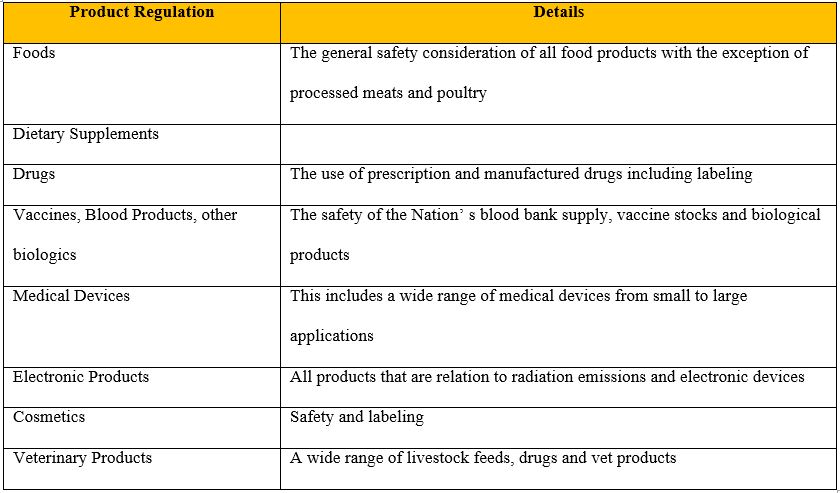
Fig 1: Illustration of Core FDA regulatory products (US Food and Drug Administration).
FDA Scope and Funding
Overall, the FDA has the responsibility for regulatory control in excess of $1 trillion of consumer goods. Of this amount, some $466 billion relates to food sales, $275 billion relates to drug regulation, $60 billion to cosmetics, and $18 billion to vitamin and natural health supplements. The FDA has a fairly wide remit authority insofar as a wide range of consumer goods are concerned. The programs for Food and Safety regulations vary widely and are covered by a legal framework that encompasses such laws as the Public Health Service Act, Food Drug and Cosmetic Act, and Controlled substances Act among others.
Regulatory Programs
The following are highlights of regulatory programs administered by the FDA.
Food and Dietary Supplements – Effectively covers the safe labelling of all types of food products sold in the US. One exception to this ruling relates to livestock and poultry processing, which falls under the remit of the Department of Agriculture. However, pharmaceuticals and medicines used by veterinarians for the treatment of animals do fall under FDA remit.
Drugs – This is split into three distinct classification of drugs (1) Generic Drugs (2) Over-the Counter (OTC) drugs, and (3) New drugs. In addition, the FDA has placed drug companies on stating they need to be aware they will be held responsible for any illegal actions performed by their executives. The FDA has a number of discretionary powers that includes banning executives from working in the pharmaceutical industry. It is expected the FDA will be issuing letters shortly to those companies and executives that have resolved legal disputes. Afterwards, exclusion letters will be sent to those found guilty of fraud or other serious misdemeanours. No doubt a number of legal challenges will follow.
Corporate Considerations
A number of the major companies, such as Glaxo in the UK, have refused to comment, particularly in light of recent scandals. “Recent fraud cases against companies like GlaxoSmithKline, ordered to pay a $750 million settlement for selling defective drugs; and Pfizer, which agreed to pay $2.3 billion for fraudulent marketing of medicine, have spurred authorities to crack down on regulation and accountability” (The Medical News). Other companies, like Pfizer, have issued statements that they have implemented new measures in prevent future occurrences, but did not issue comments on the possibility of excluding executives.
As for areas that are not specifically covered under the law, the FDA is going to take closer examination over those peripheral areas that are not automatically dealt with by law; certain actions already have an immediate exclusion posted against them under the law. “Certain crimes, such as patient abuse or a felony conviction of health-care fraud, require automatic exclusion by law, and the inspector general has the discretion to bar a person in other cases, such as a misdemeanour conviction” (Medical News Today). Because of these exclusions, it is important the FDA remain informed and up-to-date on legal issues within its scope of governance.
Works Cited
Medical News Today. “Federal Inspectors Crack Down on Corrupt Company Executives.” 10 November 2010. Web. 6 December 2012.
US Food and Drug Administration. Web. 5 December 2012.

Stuck with your Research Paper?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee

