 All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Determination of Ka of an Unknown Weak Acid, Lab Report Example
Hire a Writer for Custom Lab Report
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

Theory
The objective of this experiment is to determine the Ka of unknown weak acid by following up the pH of the acid using a pH meter. The Ph meter is used to monitor the pH of the acid during an acid based titration. The obtained pairs of data comprising of pH against the volumes of the base added in millilitres in then used to determine the dissociation constant (Ka) of the weak acid.
Theoretically, weak acids react in water solutions or with bases to form hydrogen ions and halide ions of the acid. The reaction reaches the equilibrium that can be represented by the Ka of the acid. Notably, each acid has its own Ka at a given temperature of the experiment. The value of Ka is determined by use of several methods in existence. This experiment opts to use two methods to determine the Ka of the weak acid.
The first method is the use of the pH of a solution that contains a known concentration of a weak acid. In this method, a plot of pH against volume of the base added produces an S-shaped graph that is used to establish the Ka of the acid. Picking of any two points on the graph below the equivalence point with their corresponding pH helps in calculating the Ka. The second method is the use of pH at half-neutralization point in a weak acid-strong base titration. The half pH obtained is useful in the determination of the Ka of the acid as discussed in Formulas and Equations.
Formulas and Equations

This equation is helpful in the determination of the pH of the weak acid at any given ratio of the concentration of the halide ions [A–] and the concentration of the acid [HA] at the equivalence point.
When the acid is half neutralised, then [A–] is equal to [HA] thus making the ratio of A– and HA to be equal to 1. Since the logarithm of 1 is 0, then pH = pKa at half neutralisation.
Procedure
- A 50ml beaker was obtained and rinsed with the unknown solution of the weak acid. A 10ml pipette was also rinsed with distilled water
- 10ml of the unknown acid was pipette into a clean, dry 100ml beaker and 10ml of distilled water added
- The pH of the solution was recorded at 0 volume of the base before the start of the titration.
- Both the molarity of the base (NaOH) and the initial reading of the burette containing NaOH were recorded.
- The titration was done with the addition of larger volume of NaoH (1-2Ml) followed by a 15seconds wait and then pH recorded against the volume of NaOH used.
- The volume of base being added was reduced to 2-3 drops as the reaction approached the equivalence point.
- Increments of 2-3 ml were added after equivalence point to a pH of 12.
- Upon completion of the experiment, the pH meter was rinsed with distilled water, wiped and stored.
Data and Observations
| Initial burette reading (x) in ml | Final burette reading (y) in ml | Volume of NaOH used (y-x) in ml | pH |
| 25.38 | 25.38 | 0.00 | 2.6 |
| 25.38 | 25.97 | 0.59 | 3.51 |
| 25.38 | 26.50 | 1.12 | 3.77 |
| 25.38 | 27.24 | 1.86 | 4.01 |
| 25.38 | 28.29 | 2.91 | 4.25 |
| 25.38 | 29.30 | 3.92 | 4.44 |
| 25.38 | 30.50 | 5.12 | 4.65 |
| 25.38 | 31.98 | 6.10 | 4.82 |
| 25.38 | 32.28 | 6.90 | 4.98 |
| 25.38 | 32.27 | 6.89 | 5.02 |
| 25.38 | 32.79 | 7.91 | 5.10 |
| 25.38 | 33.40 | 8.02 | 5.26 |
| 25.38 | 33.92 | 8.54 | 5.44 |
| 25.38 | 34.38 | 9.00 | 5.67 |
| 25.38 | 34.72 | 9.39 | 5.96 |
| 25.38 | 34.99 | 9.61 | 6.31 |
| 25.38 | 35.10 | 9.72 | 6.71 |
| 25.38 | 35.15 | 9.77 | 7.23 |
| 25.38 | 35.21 | 9.83 | 9.70 |
| 25.38 | 35.26 | 9.88 | 10.25 |
| 25.38 | 35.34 | 9.96 | 10.46 |
| 25.38 | 35.36 | 9.98 | 10.61 |
| 25.38 | 35.45 | 10.07 | 10.72 |
| 25.38 | 35.56 | 10.18 | 10.88 |
| 25.38 | 35.65 | 10.27 | 10.99 |
| 25.38 | 35.76 | 10.38 | 11.08 |
| 25.38 | 35.90 | 10.52 | 11.16 |
| 25.38 | 35.96 | 10.58 | 11.21 |
| 25.38 | 36.19 | 10.81 | 11.31 |
| 25.38 | 36.43 | 11.05 | 11.39 |
| 25.38 | 36.56 | 11.18 | 11.45 |
| 25.38 | 36.85 | 11.47 | 11.51 |
| 25.38 | 37.16 | 11.78 | 11.59 |
| 25.38 | 37.46 | 12.08 | 11.65 |
| 25.38 | 38.25 | 12.87 | 11.76 |
| 25.38 | 39.25 | 13.87 | 11.86 |
| 25.38 | 40.16 | 14.78 | 11.93 |
| 25.38 | 40.64 | 15.26 | 11.97 |
| 25.38 | 41.64 | 16.26 | 12.01 |
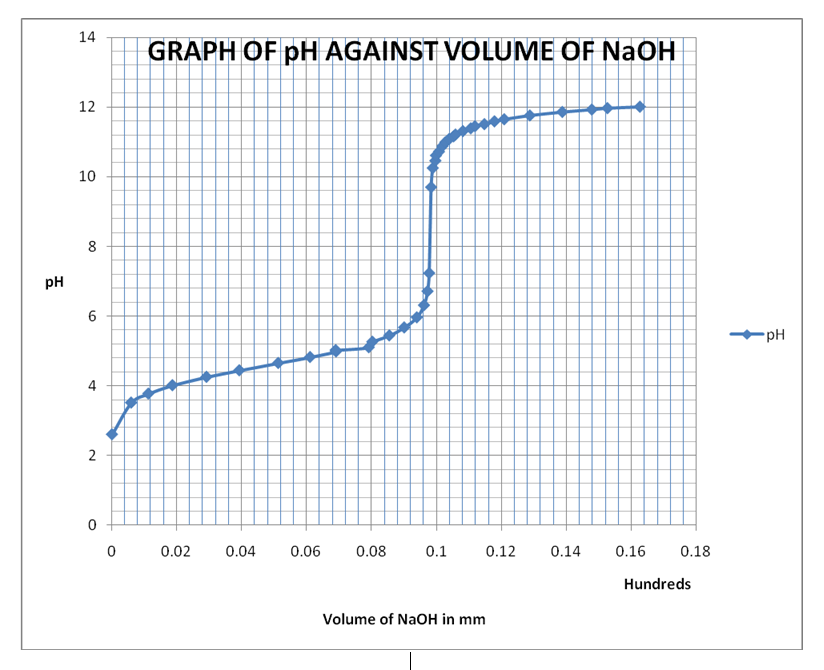
Calculations
From the graph, the equivalence point is at approximately pH 8.2 and at volume 10.00mm of NaOH used.
Moles of NaOH used at the equivalence volume is calculated as:
Moles = volume of NaOH used × molarity of NaOH
= 0.000997 moles.
Therefore, moles of the acid in 10ml used = 0.000997 moles.
Molarity of the acid = (1000 × 0.000997)/10
[HA] original = 0.0997M
Note: 10ml acid was diluted by adding 10ml water before titration. Therefore, the concentration of the acid used to calculate Ka is half the original concentration.
Therefore, [HA] diluted = 0.0997/2
[HA] diluted = 0.04985
Determination of Ka
Method 1: using the pH of the diluted solution
At a point on the graph of pH 4.8, volume of base = 6ml
Remember, pH = pKA + Log [A– ]/[HA]
4.8 = pKa + Log [A– ]/[HA]
Concentration of HA
Volume of base used = 6ml. molarity = 0.0997M
Moles = 6 × 0.0997/1000= 0.0005982
pKa = 4.8 – (Log 0.0005982/0.04985)
pKa = 4.8- – 1.92
pKa = 6.72
pKa = -(log)Ka
-6.92 antilog = Ka
Ka = 12.0 ×10-6
Method 2: half-neutralisation point
pH1/2 = pKa
From the graph, pH1/2 = 4.1
Therefore, pKa = 4.1 = -(log)Ka
Ka = 10-4.1
Ka =10-6×100.9
Ka = 7.94×10-6
Discussion and Conclusion
The results indicate that monitoring the pH of a titration reaction is equally an important and accurate way of determining the dissociation constant of an acid. The experiment was successful since it enabled the calculation of the Ka of the unknown week acid HA although results from method 1 slightly differed with those from method 2. However, the accuracy of the results can be improved if both human and technical errors are minimised. Human errors result from the incorrect reading of the burette volume due to parallax effect. Technical errors arise from readings from the pH meter, which are at times inconsistent in a single data reading.
In conclusion, the Ka of the weak acid was determined successfully upon monitoring of the neutralisation reaction between a weak acid (HA) and a strong base (NaOH).

Stuck with your Lab Report?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee

