Dialysis Tubing Experiment, Lab Report Example
Abstract
The purpose of the lab experiment was to determine the type of substances that can flow through the pores of a dialysis tubing and to demonstrate an example of a selectively permeable membrane. The tubing is used in the experiment to act as a cell membrane that allows water and other solutes to diffuse readily through the semi-permeable membrane. Despite the fact that there are pores in the dialysis tubing, they are tiny to enable bigger molecules, such as sucrose to pass through. Therefore, it functions as a plasma membrane of living cells because it only permits specific molecules to diffuse directly through a phospholipid layer. All the solutions in the lab experiment were prepared with some concentration of solute and solvent. The trials that had a solution in the dialysis tube and a different solution should outside yield no change in the dialysis tubing.
Lab 4: Dialysis Tubing Experiment
Introduction
Every living cell has a plasma membrane that encloses a plant or animal cell. It forms a boundary that mainly separates a plant or animal cell from its surrounding. However, a cell membrane not entirely impermeable because it is porous; hence it is regarded as selectively permeable. Due to its permeability, a cell membrane controls the nature and type of molecule that passes through it. For instance, in all living cells, some mechanisms involve the movement of materials, such as waste substances and other materials that move and leaves the cell (Regenstein, Regenstein & Kochen 2014). In its usual processes, the cell takes in oxygen that it manly uses for respiration and also engages in other activities, such as expanding carbon-dioxide. The cell membrane is the part of a living cell that regulates the concentration of ions by allowing them to move through a plasma membrane. Apparently, in any of the cases, a cell membrane is considered to be selectively permeable since it permits.
Water is an example of substances with smaller molecules that easily moves through the porous cell membrane. The movement of solvent molecules takes place due to the differences in concentration (Lee & Lee, 2003). In essence, water usually moves from an area where the concentration is high to a region with low concentration through a process known as osmosis. The simple biological fact is that when the concentration of dissolved particles is higher, the concentration of water molecules is lower, hence enabling them to diffuse (Regenstein, Regenstein & Kochen, 2014). In simple terms, osmosis may be defined as the diffusion of solvent molecules, such as water, through a semi-permeable membrane to a hypertonic area. For this reason, when the solvent molecules pass through a cell, the movement is usually is usually accomplished through osmosis. The flow of water through a semi-permeable membrane of a plant or animal cells is considered as the simplest case of osmosis.
Researchers, especially molecular biologists consider osmosis in different perspectives. However, the most common definition of osmosis the movement of solvent molecules, for instance, water, from areas of concentration to low concentration. The biological process usually takes place through a semi-permeable membrane. The solvent molecules move from a higher solute concentration towards a direction, which will eventually equalize the solute concentration. Osmosis is a natural process that can also be used to describe the physical processes where the solvent molecules are transferred through a cell membrane. Notably, it is the solvent that has to be permeable to the solvent and not the solute. According to Ramlingam (2008), the cell membrane separates two solutions that have different concentrations. It describes a physical process where solvent molecules, such as water are transferred across a semi-porous membrane (Ramlingam, 2008).
In the lab, experiment water was used as the solvent. It is apparent that when the cell is submerged in a solvent, the semi-permeable membrane will allow commencement of the diffusion process. On the same note, diffusion can be defined as a biological process that causes diffusion of solvent molecules or atoms through a semi-porous membrane. As mentioned, the operations take place due to the difference in concentration, that is it takes place from a high chemical potential ara to an area of low concentration or rather a low chemical potential region. In other words, diffusion is the movement molecules as a result of a concentration gradient (Ramlingam, 2008). Most studies that focus on living cells suggest that a semi-permeable membrane permits only selected substances through it.
A semi-permeable membrane is part of a cell. However, for research purposes, the biological or synthetic material may be used, such as the polymeric membrane that permits only individual molecules or ions to pass through. In most lab trials, a dialysis tubing is utilized as a separation technique. It is composed of materials that readily enables the transportation of small molecules based on a diffusion gradient (Todd, 2012). Therefore, dialysis refers to the transfer of a solute across a porous or selectively permeable membrane. In practical applications, dialysis is a process that s used to separate a solution or a mixture of different substances or molecules as a result of the differences in their molecular sizes. In the process, a cell is bathed in a solution, and it involves passive procedures, which do not necessarily require expending on energy on the part of a cell. In most cases, the process is driven by natural physical means due to the presence of free energy.
Most of the physical and chemical reactions that take place in the cell are considered as the basis of life. The energy available to undertake any activity is known as free energy and is directly influenced by concentration, pressure, and temperature. Free energy enables molecules to move from regions of higher concentration to low concentration pressure and temperature. The biological process involves a passive movement of particles, regarded as diffusion. The presence of a gradient is the difference between the higher concentration and the lower concentration. Smaller molecules are transferred or diffuse from higher to lower free energy to an equilibrium level. Therefore, smaller molecules can easily diffuse in air or liquid. The movement can also take place across plant and animal cell membranes.
The most common type of biological diffusion is osmosis. It is a biological system where a solvent diffuses across a semi-porous cell membrane (Stillwell 2013). In plant and animal cells, water molecules are transferred, and the process is accomplished through osmosis. A membrane structure causes selective permeability. During the process, a cell changes materials within its surrounding, a process which is controlled by the plasma membrane.
This experiment shows how the cell membrane (dialysis tubing) will allow the solvent to pass through the cell membrane (dialysis tubing), but stop larger solute molecules like sucrose, which will result in hypertonic, isotonic and hypotonic cells. When a cell is hypotonic, water enters by osmosis, and the cell tends to swell. Animal cells in a hypotonic solution may expand to the point of bursting (Anestis 2007). Organisms that live in surroundings that contain salts or other molecules at higher concentrations than their bodies must continuously expend energy to replace water lost by osmosis.
In the situation mentioned, the outside solution is said to be hypertonic to the cells (hyper àover or above). The concentration of water inside and outside of cells is usually equal or isotonic (isoà the same). Based on the explanation, to ensure that the fluids are on either side of the plasma membrane, then when the animal cells are isotonic they should continuously use energy. The energy is usually used to pump Na ions through a process known as active transport. However, failure to do this would mean that water moves inwards. As a result, the movement of water molecules within the cell may cause it to burst. It was hypothesized that during this experiment the dialysis tubing used to replicate cells would change tonic states depending on the solute and solvent.
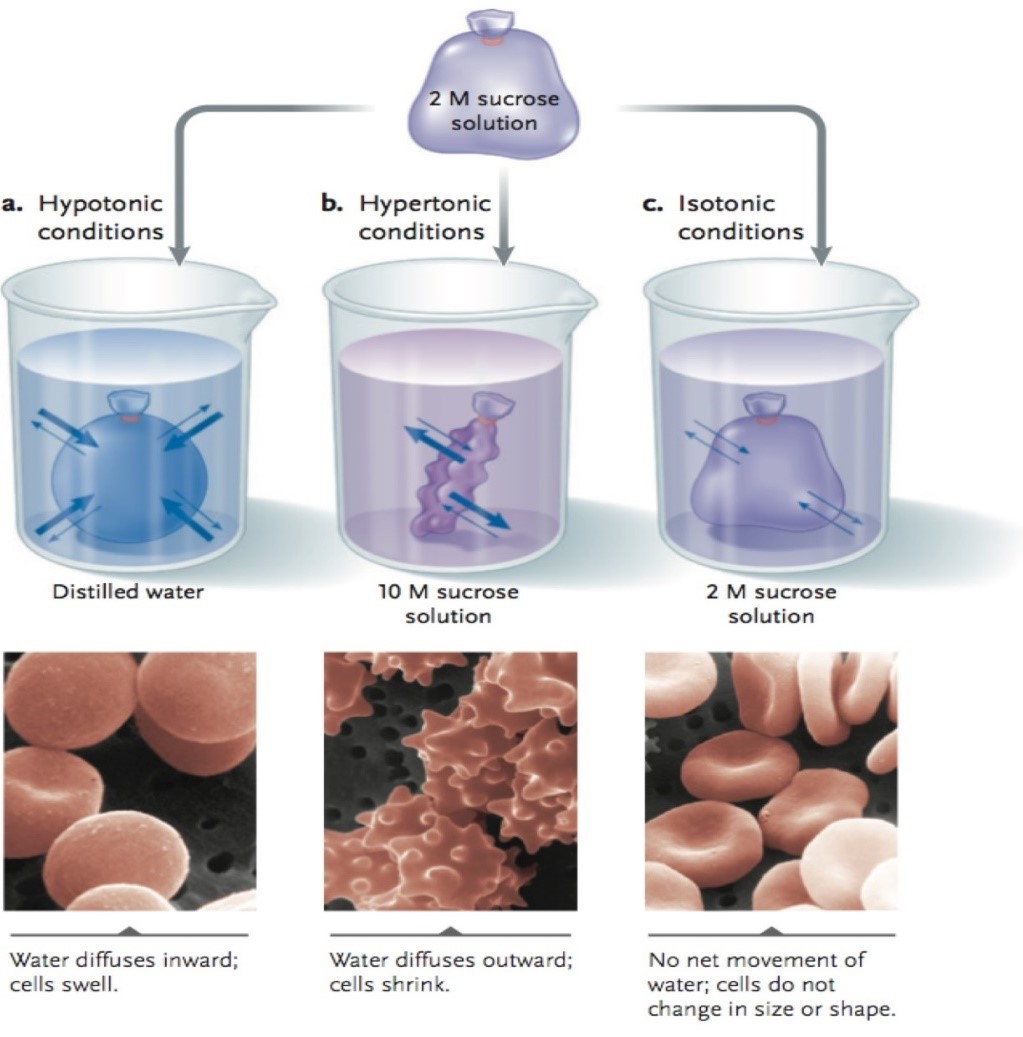
Figure 1: Tonicity and osmotic water movements
Through diffusion, molecules show a tendency to spread out in larger spaces. The biological process can efficiently demonstrate through observing the action observed in the movement of water molecules (Lee & Lee, 2003). Ideally, water molecules are small in size but are usually in constant motion. The movement of molecules is deemed to be random. Hence it is necessary to understand that the random movement of water molecules may continue even after reaching the equilibrium (Pack, 2007). The experiment will use dialysis which is a process used to separate smaller molecules from larger molecules through a semi-porous membrane, to make the point clear. Additionally, in the study, the tubing was mainly used to simulate semi-permeable cell membrane (Lee & Lee, 2003). As mentioned before, the dialysis tubing is made up of selectively permeable materials, preferably cellulose that has microscopic pores and is perforated. Mainly, the pores at the surface of a dialysis tubing are small enough to be able to model the cell membrane concerning the molecular sizes, which may not readily diffuse through the material.
Materials and Methods
In the process, three 250 Ml beakers were labeled each containing a different solute that was made of 250 g sucrose dissolved in 250 mL. The solute was transferred to beakers #2 and #3 until they were ¾ full. Water was then added to beaker #1 approximately ¾ full. After preparing the solute solutions, three-segment dialysis tubing was cut about 10 cm long each and submerged in the water until it began to unfold. Each piece has one end folded back on itself approximately 1 cm and the end tied tightly using a thread. Each of the three tubes was then filled as follows; tube labeled one was filled with sucrose solution, tube labeled two was filled with sucrose solution, and tube marked three was filled with water. Further, the open end of the tubes was folded and tied as the previous side. The last procedure comprised of placing tube labeled 1 in beaker marked 1, tube labeled 2 was placed in the beaker labeled 2, and tube labeled 3 was placed in the beaker labeled 3.
Results
At the start of experiment 8:04 pm, the set-up of the apparatus was as follows:
Beaker labeled 1 was filled with water and dialysis tube labeled 1 was filled with sucrose solution. The tube used was flimsy and could quickly expand.
Beaker labeled 2 was also filled with water, and the dialysis tube labeled 2 was also filled with sucrose solution. Similarly, the tube used was flimsy and could expand more easily. The liquid that was used was transparent but appeared cloudy.
Beaker labeled 3 had sucrose solution, and dialysis tube labeled 3 was had water solution. The tube that was used was flimsy and could expand more easily. The liquid used was transparent but appeared cloudy.
At the end of experiment 11:04 pm, the set up of the apparatus appeared as follows:
At the end of the trial, in the dialysis tube labeled 1 had water, the tube demonstrated a hypotonic cell. It expanded and absorbed water molecules; hence causing it to swell. The liquid that was used was transparent.
In dialysis tube labeled 2 filled had sucrose solution, the tube demonstrated an isotonic cell. The tube was flimsy and could expand more easily. The liquid was transparent but cloudy.
Dialysis tube labeled 3 was had sucrose solution. The tube demonstrated hypertonic cell. Also, the tube was extraordinarily depleted and shriveled.
The following results were obtained after treatment were as follows;
Dialysis cell after 1 hour 9:04 pm
In the model, the cell labeled 1 the dialysis cell sunk to the bottom of the beaker. Bubbles formed on the inside of the beaker but when compared to cell marked 3, the cell marked 1 had swelled slightly.
In model cell#2, the dialysis cell repositioned itself centered with the beaker. Additionally, the cell had not swelled or shrunk.
In model cell# 3, dialysis cell remained floating at the top of the solute. Also, the cell began to deflate or shrink.
In dialysis cell after 2 hours 10:04 pm
In model cell #1, dialysis cell had swell and expanded. Some bubbles remained on the inside of the beaker, and the cell submerged 100%.
In model cell #2, the dialysis tube was diagonal inside the beaker. However, the cell had neither expanded or shrunk. Also, the cell submerged 100%.
In model cell#3, the dialysis tube remained floating on top of the solute. It was also noted that the cell appeared shrinking and the solute was pooling with a slight percentage remaining outside of the water.
In the dialysis cell after 3 hours 11:04 p.m.
In model cell #1, the dialysis tube had reached its peak swelling. It demonstrated a hypertonic solution effect on a cell.
In model cell#2, the dialysis tube was diagonal inside the beaker. The cells appeared to neither expanded or deflated. Furthermore, it demonstrated an isotonic solution on a cell.
In model cell# 3, the dialysis tube seemed to submerge just below the surface of the solute. The cell appeared extremely deflated, as well as a hypertonic solution on a call.
Discussion
The experiment modeled an osmosis process using dialysis tubing. From the results, the hypothesis suggested was right. The dialysis tubing was permeable to smaller molecules. However, larger molecules such as sucrose solution were not allowed to pass through the cell membrane. Notably, this explains the availability of reducing sugar in the mixture. The experiment was conducted to examine the permeability of dialysis tubing to a sucrose solution (Yan, 2003). Plant and animals cells usually depend on the biological processes they need other materials from their surrounding and release waste products to the environment (Allen & Harper 2011). They have membranes that are made using a phospholipid layer embedded between different substances. Due to the semi-permeability of the cell membrane, it controls the flow of other substances. Moreover, the porous membrane allows others materials that have smaller sizes to pass through, and in any cell, the property is known as permeability.
Living cells have a characteristic of selective permeability that enables it to control molecules that can pass through. The property allows cell membranes ensure that smaller molecules move and inhibit larger molecules from passing through. The tubing used is mainly used in several experiments as a separation techniques. The tubing is necessary when demonstrating various biological processes, such as diffusion, osmosis, and other molecular movements across the cell membrane (Yan 2003). Also, the cell membrane is used to separate dissolved substances that have different molecular sizes. Though, some materials are considered too quickly through the semi-porous membranes of the dialysis tubing while others are not allowed to pass through the semi-permeable membrane.
In the experiment, the selective permeability of the tubing to sucrose solution was tested. The hypothesis predicting that the cells would change tonic states was proved true because the tubing acted as a semipermeable membrane. It permits only specified molecules to pass in and out through the cell membrane. Inside each cell and beaker, different solutions were used to test various outcomes. Beaker one with water and cell another with sucrose solution, which showed the most rapid change. The cell began to swell and expand quickly resulting in a hypotonic cell. Beaker two, full of the sucrose solution and tube two full of the sucrose solution had no change at all. The concentrations within the beaker and cell were balanced, and therefore no diffusion was necessary, thus resulting in an isotonic solution/cell. Beaker three, full of sucrose solution and cell three full of water showed the inverse of beaker/cell one. Cell three began to shrink and deplete until there was not much solute left in it at the end of the experiment; this process demonstrated and hypertonic solution.
Throughout this experiment, the things established were that water(osmosis) moves(diffuses) from regions with high free energy content to areas with low free energy content through a cell membrane with an attempt to equalize concentrations. While performing this diffusion through osmosis, some things can happen to the cell such as becoming hypertonic(depleted), as shown in experiment cell three, isotonic(standard), as shown in experiment cell two, and hypotonic (swollen), as demonstrated in experiment cell one (Allen & Harper 2011). The experiment was successful in describing three different cell states and how some molecules can pass through membranes while others are blocked entry.
About living cells, the current study modeled what would happen to animal and plants cells. In the case of animal cells, the cells may be stated to be in an isotonic condition, which s ideas for the proper functioning of the body through offering stability and maintaining biological systems (Schleif Wensink 2011). When is a hypotonic mixture, animal cells may burst while in a hypertonic solution, the cell will shrink or wither; hence making the cytoplasm dense and the contents may be concentrated within the cell, which might render it to die eventually. In the case of a plant cell, the researcher observed that the ideal solution is a hypotonic solution. In such situation, the plasma membrane expands to reach the limit of the cell wall. When such cases occur, the cell may burst or in other words, lyse (Stillwell, 2013). Besides, in a plant cell the condition is regarded as hypertonic to its surrounding; hence the water molecules will enter into the cell until it reaches a limit where the internal pressure will eventually prevent further influx.
Apparently, to maintain such a balance of water and the solutes if crucial for the proper functioning of the cell because it assists in maintaining the balance when the fluid becomes isotonic. Similarly, the extra fluid may be hypertonic or isotonic depending on the concentration of the solution. The process may channel the water to leave the plant cell and causes a loss of the turgor pressure (Schleif & Wensink 2011). Most often, the loss of water in the plant cells is regarded as wilting. Consequently, when a plant cell experiences hypertonic conditions, most often they detach from the wall and constrict the cytoplasm. The biological term the process is plasmolysis.
It is crucial to understand what substances can permeate the cell membrane because such chemicals are ideal for cell functioning. Similarly, the cell should have a semi-permeable membrane because it helps to safeguard against harmful substances that want to enter the cell (Stillwell 2013). It is also necessary to understand how water molecules are transported through the cell membrane through osmosis because when a solute concentration is unregulated, the end product is known as net osmosis, which takes place inside or outside a cell; hence resulting into cytolysis or plasmolysis. Therefore, the plasma membrane of a cell can be modeled n several ways. However, dialysis tubing is very helpful when modeling the substances, which will diffuse or transported out of a cell membrane.
Dialysis tubing is manufactured from regenerated cellophane or cellulose to ensure that molecules have a filtered flow and that larger solute molecules do not enter into the dialysis tubing. Therefore, dialysis tube functions like a cell membrane that allows small molecules to permeate through the membrane (Schleif & Wensink, 2011). Therefore, the function of a dialysis tubing mimics a cell despite the fact that it has a different structure.
From the experiment, it is apparent that dialysis is functioned as a cell membrane. For this reasons, it is also regarded as a type of a selectively permeable membrane due to the presence of microscopic holes or pores on the surface. The semi-permeable nature of the dialysis tubing s a characteristic that enables to separate substances or molecules depending on their sizes. Hence, molecules that are smaller in size than the pores may quickly pass through the dialysis tubing (Schleif & Wensink, 2011). Consequently, larger molecules are not allowed to pass through the tubing and are trapped within or outside the cell. The tubing is available and may be ordered from scientific or laboratory supply companies. The tubing is also available in different pore sizes so that any experiment involving separation of substances that have varying molecular sizes can easily be carried out.The tubing is usually used in molecular and biochemistry laboratories to separate materials that are in complex mixtures.
References
Allen, C., & Harper, V. 2011. Laboratory Manual for Anatomy and Physiology. Hoboken, NJ: Wiley.
Anestis, M. 2007. Five steps to a 5: AP biology. New York: McGraw-Hill.
Lee, Y. C., & Lee, R. T. 2003. Recognition of carbohydrates in biological systems: Part B. Amsterdam: Academic Press.
Pack, P. E. 2007. CliffsAP biology. Hoboken, NJ: Wiley Pub.
Ramalingam, S. T. 2008. Modern Biology. Onitsha: African First Publishers.
Regenstein, J. M., Regenstein, C., & Kochen, B. 2014. Food protein chemistry: An introduction for food scientists. Orlando: Academic Press.
Schleif, R. F., & Wensink, P. C. 2011. Practical Methods in Molecular Biology. New York, NY: Springer New York.
Schleif, R. F., & Wensink, P. C. 2011. Practical Methods in Molecular Biology. New York, NY: Springer New York.
Stillwell, W. 2013. An introduction to biological membranes: From bilayers to rafts. Amsterdam: Elsevier/Academic Press.
Stillwell, W. 2013. An introduction to biological membranes: From bilayers to rafts. Amsterdam: Elsevier/Academic Press.
Todd, I. S. 2012. Dialysis: History, Development, and Promise. World Scientific Publishing Co Pte Ltd.
Yan, Q. 2003. Membrane transporters: Methods and protocols. Totowa, NJ: Humana Press.

Time is precious
don’t waste it!

Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee






