 All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Electric Field and Electric Potential, Lab Report Example
Hire a Writer for Custom Lab Report
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

This is an electric field and electric potential lab. We conducted a comprehensive experiment that proved a valid existence of the relationship between equipotential lines and the electric field lines. It was found that the slope of the linear fit depicts that the electric field runs perpendicular to the potential lines, whose electric field is uniform.
Also the Validity of Gauss’s law was also put to the test. By measuring the E-field outside the outermost cylinder, the result was found to be consistent with Gauss’s Law.
In this lab we studied the electric fields between two parallel metal bars and using a water bath we mapped out the equipotential, plotted the electric fields and determined the electric field.
This helped us understand the relationship that exists between the equipotential lines and the electric field lines.
We determined the electric potential that is produced by a given set of electrodes that are held at a fixed voltage.
We will be experimenting on the validity of Gauss’s law and the principle of superstition.
We created the experimental set-up according to the diagram that was provided in the lab guide.
- AC source
- Water Tank
- Electrodes
- Digital Multi-meter
- Single-Point Lead Probe
- Two-Point Lead Probe
- Conducting Ring
- Coaxial Rings (different sizes)
We created the experiment as had been provided in the lab guide.
We used an AC source to generate the field in the water tank as a DC source would result in the build-up of charge near the electrodes.
We used the Digital Multi-Meter to measure the RMS voltage drop between a selected fixed location and a number of various locations in the tank.
Using the single lead probe, we measured the potential of a number of equally spaced points in between the two electrodes.
We then recorded the voltage measurement from the DVM directly on a sheet of paper
In GA, we made a graph of voltage V vs. distance x from the grounded bar.
We then added the conducting ring in the centre of the two bars and drew appropriate equipotential lines (error for this part was ignored).
We then used the signal generator to generate an AC signal between the two bars.
We picked one of the two bars as the fixed location for the black lead from the digital multimeter. This was the selected location at which the potential is zero.
We used the red digital multimeter lead to measure RMS voltage at various location within the tank.
We then switched to the two-point probe and checked the electric field outside the ring at three locations.
We rotated the probe until the DMM gave the largest possible RMS. This was to determine the direction of E.
Turn of the signal generator and remove the bars and the ring from the tank
Set up a pair of oppositely charged coaxial rings in the water tank (i.e. the largest and the smallest rings available)
Connect the leads for the signal generator directly to the rings using the alligator clips.
Make predictions of whether the cylinders themselves will be equipotentials.
Make measurements to test prediction
Using the inner ring as location of zero potential, measure the RMS voltage at 5 different distances measured from the common axis. All the five distance are larger than the radius of the ring and smaller than the radius of the outer ring.
For each distance make measurements of the distance r, (measuring Vrms) at angles of 0°, 90°, 180°, and 270° from the positive X axis.
Compute the average value for Vrms for each distance.
Tabulate and graph these results.
Switch to the fixed spacing probe assembly and make measurements of the electric field in between the two circles.
Measure the electric field every 1.0 cm along two different direction –x and y.
Record the E values as well as the distances r from the centre of the circles, choosing the r value at a point exactly in the middle of the probe tips.
Enter the measured E values and the corresponding r values in a graph. Plot E vs. 1/r making use of the calculated column option as needed.
Use the slope of the straight-line relationship to make determination of the charge per unit length of the inner ring and its error.
We then measured the E-field outside the outermost cylinder.
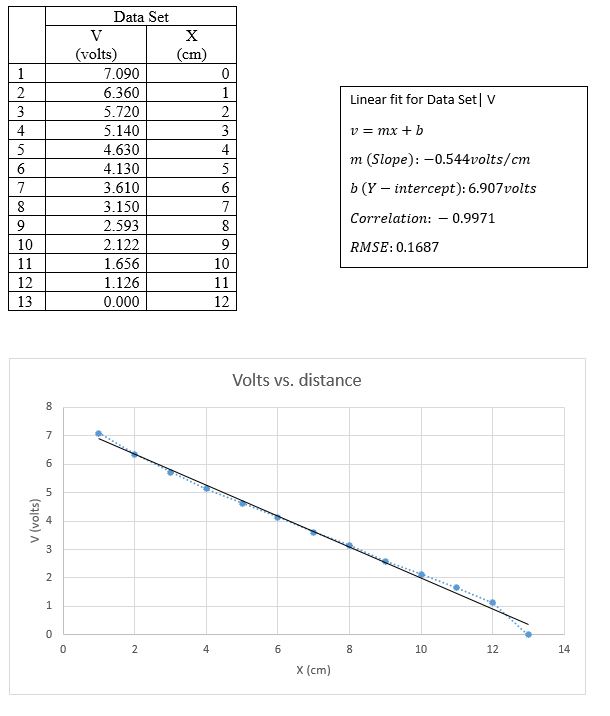
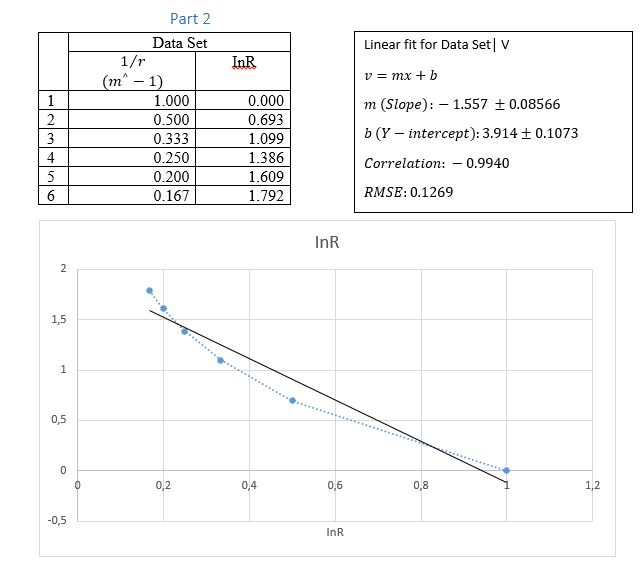
Results
The slope of the linear fit depicts that the electric field runs perpendicular to the potential lines, whose electric field is uniform.
The electric field strength inside the ring is zero.
Since work is zero, force is perpendicular to motion. Force in this case is in the same direction as E. therefore, motion or the direction of motion is perpendicular to E.
The RMS measurements inside the ring confirm that the field inside the ring runs perpendicular to the potential lines, hence the RMS recordings inside the ring have a negative value as compared to the ones outside the ring.
After measuring the E-field outside the outermost cylinder, the result was found to be consistent with Gauss’s Law:
Using point 0.500

The fact that E is proportional to 1/r is consistent with the linear dependence of the electric potential on In(r). The electric field is directly proportional to the inverse of the distance from the centre of the wire to the field point. This is because the distance from the centre of the wire determines the strength of the electric field as the field are generated or originate from the centre of the wire. Tis same concept is applied in the electric field of the inner ring as it is described by the potential difference between the inner ring and the second point at the same distance r.
The experiment was a success as it proved there is a valid existence between the equipotential lines and the electric field lines. This was proven by the slope of the linear fit, which depicted that the electric field runs perpendicular to the potential lines, whose electric field is uniform. The electric field strength inside the ring was found to be zero and since work is zero, force is perpendicular to motion. Force in this case is in the same direction as E. therefore, motion or the direction of motion is perpendicular to E.
The Validity of Gauss’s law was also proven with measuring the E-field outside the outermost cylinder. The result was found to be consistent with Gauss’s Law:
Using point 0.500


Stuck with your Lab Report?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee

