All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Gel Filtration Chromatography, Lab Report Example
Hire a Writer for Custom Lab Report
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

What is the purpose of the slow loading procedure from steps 1- 4? Why do we put small quantities of buffer on top of the column?
The mobile stage or eluent is composed of an unadulterated solvent or a combination of distinct solvents. It is selected so that the retention index of the compound being evaluated is 0.2- 0.3. This is performed for the eluent to complete the chromatography (MSU 1).
Why must the column never run dry?
The flow rate must be maintained. More rapid flow rates can be attained by the application of a compressed gas cylinder or pump (MSU 1).
What has to be done to the column fractions before they are assayed?
The dry mass of the solute and the stationary mass must be placed in proportion (MSU 1).
How will you know when it is time to stop running buffer through the column at the end of the experiment?
In the circumstance of the blue dextran and the DNP- aspartate, there are yellow and blue molecules which can be observed eluting from the columns (MSU 1).
Objective
The objective of the experiment is to classify proteins which are derived from a complex mixture by the application of the gel filtration chromatography technique. This approach is applied by biochemists in the cases that the proteins are more expansive than the parts are not included in the column. The molecules which are smaller elute last and then are gathered in cuvettes for review by the spectroscopic perspectives. An unknown protein will be identified during the lab.
Introduction
The procedure where analytes are separated as a result of the varying dissemination between two phases (stationary and mobile) is delineated as chromatography. The compounds that are migrating in the mobile phase have interactions with the stationary phases. The compounds that are retained during the stationary phase migrate slowly, while the compounds that interact faintly migrate more rapidly (Steigel 118; Walsh 45).
Background
An approach that has been widely applied by empiricists for over three decades, the gel filtration chromatography performed the purification of the biological samples in accordance with their molecular dimensions and mass. The process incorporates a beaded column, which is composed of diverse pores that enable the densest molecule to become absorbed through the columns; the elution initially begins while diminishing in dimensions and mass. The proteins that are globular manifest distinct indexes of migration. This is attributed to the premise that the most massive proteins do not have the capacity of entry into the conduits of the interior pores (Burden & Whitney 98). Consequently, the eluent does not have the capacity of readily moving rapidly in a downward direction in the column. The molecules that have smaller dimensions have the ability of migrating through the pores. The molecules that have sizes which are identical to the diameter of the pores will require more time in order to pass through the pores in the column (Steigel 45).
Gel filtration is a technique that can be easily applied that facilitates the biochemists’ analysis of biological samples. The analyses are conducted in order to assess the molecular masses and for the purposes of purification. The proteins that have a globular quality, those include DNA, enzymes, phenol, antigens and urea. The proteins were gathered into the proportions and subsequently reviewed by the spectrometer for the classification of which of the globular proteins is the eluent composed (Walsh 118).
Procedure and Materials
Materials
- Column peripheresis.
- Chromatological columns.
- Separation compound, Composed of 0.006 g of blue dextran and 0.006 g DNP- aspartate. Twelve milligrams of the mystery protein had been included in 1 ml of elution buffer.
- Elution buffering solution of HCl =0.05 M with a pH quality of 7.5.
- Safety Goggles.
Procedure
- The buffer had been drained that was located on the upper part of the column. This process was carried out until the meniscus had been initiating entry into the resin. A graduated cylinder had been placed beneath the column in order to retrieve the effluent.
- Approximately half of a milliliter of the separation had been placed into the column. Care had been exercised in this stage of the experiment. The loading procedure had been performed slowly and deliberately. A pipet pump had been applied to the Pasteur pipet.
- The mixture was allowed to run in the column while the effluent was being gathered into the graduated cylinder.
- There was a limiting of the addition of buffer to the point where the mixture had comp0reheisvely entered. Subsequently, small amounts of the buffer had been added and the column was permitted to run.
- As the mixture entered safely into the portion of the gel, which had been approximately two centimeters from the upper part of the gel bed, the column had been replenished with elution buffer. The top of the gel bed was not permitted to desiccate in order to maintain the rate of flow of the effluent.
- As the blue dextran was approximating egress from the column, the graduated cylinder was extracted and the volume that had been collected was documented. Subsequently, fractions were gathered into one milliliter cuvettes,
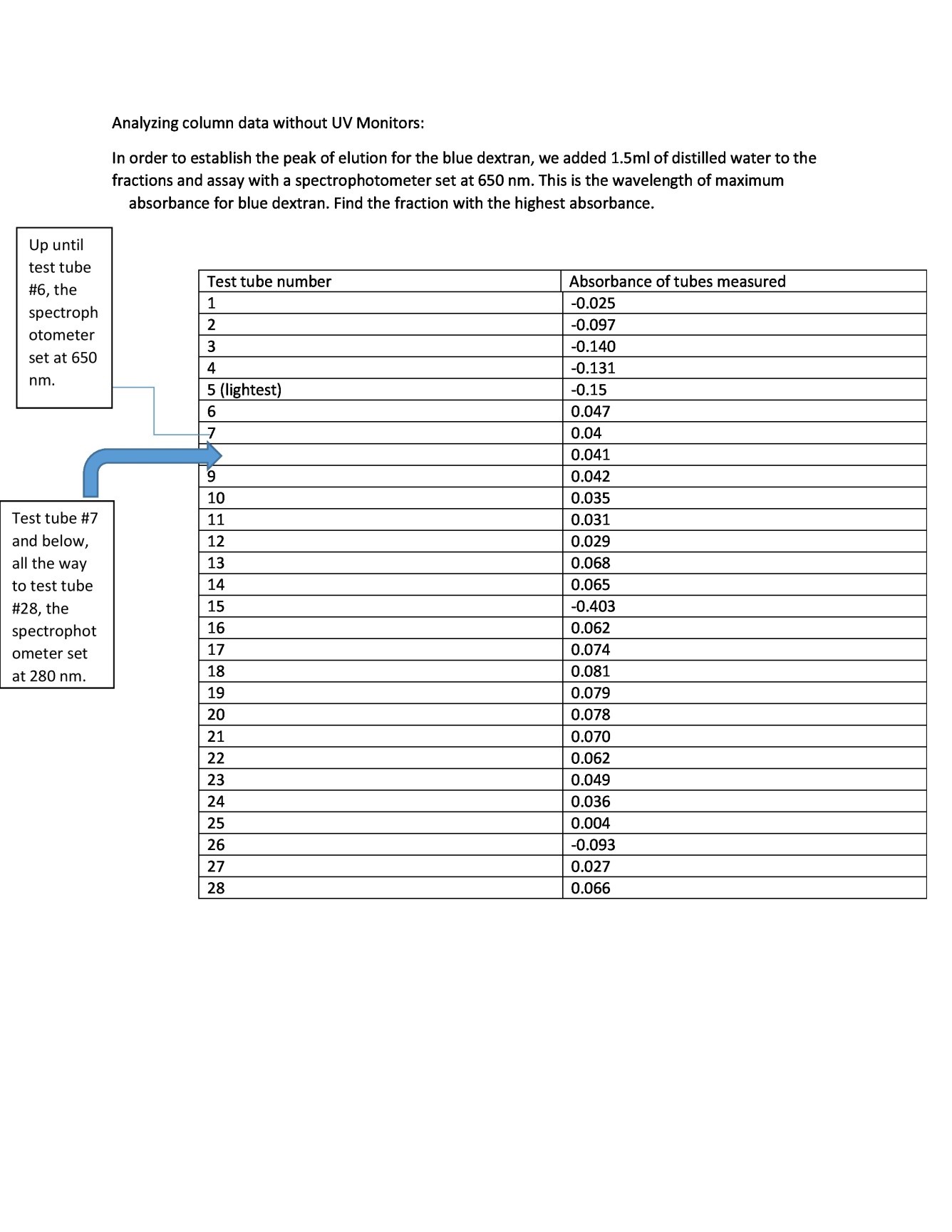
Analysis of the Information in the Absence of UV Monitors
- In order to establish the peak of the process of elution for the blue dextran, a milliliter and a half of water had been added to the assay and the fractions. The spectrometer was established at 650 nm. The fractional position with the highest absorption had been found.
- The elution had been continued and the peak of the mystery substance had been detected by assessing the absorbance of the cuvettes at 500 nm. Considering that all proteins possess a maximum index of absorbance of 280 nm, the mystery substance emitted a reddish brown color and had been found with observable spectrophotometry.
- The elution had been added to the column and the fractions were collected until the DNP- aspartate was totally eluted and the column was clean. The p3eak elution proportion had been encountered by assessing the absorbance at 440 nm subsequent to the addition of a milliliter and a half of water.
Table 1: Absorbance at 650 Nm for Test Tubes 1- 6 and Absorbance at 650 Nm for Test Tubes 7- 28.
Analysis of Results Questions
On a single graph, plot the absorbance of the fractions versus the volume taken at that fraction. For example, if you collected 20 ml into the graduated cylinder and then began collecting 1 ml fractions and your first dextran blue fraction that had an absorbance on the fourth fraction , then plot the absorbance versus 20 ml + 4 ml = 24 ml.
What are the elution volumes that give the highest absorbance for the blue dextran, the unknown protein and the DNP – aspartate?
The absorbance of Blue Dextran 530 nm has a cumulative volume of 1.8239g.
Absorbance of the mystery protein has a frequency wavelength of 596 nm with a cumulative content of 64.38g.
Absorbance of the DNP – aspartate has a frequency wavelength of 650 nm with a cumulative volume of 1.795 g.
Calculate the Kd value for the unknown protein.
– 0.003.6189/ 0.068- 0.064.38 = 0.17789 = K
Empirical value is 15.60 ± 0.73 (Bojesen & Bojesen 770). Difference between empirical value and laboratory value for the Kd has a difference of 9.2 %.
Use the information in table 2 to make a graph of the log10 MW versus K
Interpolate from the graph and determine which molecular weight of the unknown protein. You do not need to determine its identity.
| Protein | Molecular Weight (g/mole) | Log10 Molecular Weight | Kd |
| Trypain inhibitor (pancreas) | 6,500 | 3.81291336 | 0.70 |
| Trypain inhibitor (lima bean) | 9,000 | 3.95424251 | 0.60 |
| Cytochrome c | 12,400 | 4.0934217 | 0.50 |
| ?- lactalbumin | 15,500 | 4.19033170 | 0.43 |
| ?- chymotrypain | 22,500 | 4.3521825 | 0.32 |
| Carnbonic anhydrase | 30,000 | 4.47712125 | 0.23 |
| Ovalbumin | 40,000 | 4.68124123738 | 0.12 |
Questions
Why do large proteins come out of a gel filtration column faster than small ones?
In the gel filtering chromatography, the polyacrylamide beads that have a microscopic quality with small pores are arranged in a column. A protein sample is applied to the top of the column and migrates through the column possessing the solvent. As the sample migrates through the solvent, the smaller molecules are passess thriogh the openings while taking a longer route than the molecules that do not have the capacity of enetering the beads and moving around them. Consequently, proteins are separated according to their dimensions with the more expansive molecules moving through the column faster than the smaller ones.
Doesn’t it make more sense for small things to move quickly through a gel?
Yes, it does and in the case of agarose gel that would be the case. In gel filtration chromatography, the more expensive proteins are available in the initial fractions while the smaller molecules of protein are available in the later ones (MSU 1).
Can you use Sephadex G- 75 to separate alcohol dehydrogenase (MW 150,000) from ?- amylase (MW 200,000)? Why or why not?
Neither of the molecules would be able to get through. This would be resulting from the low exclusion limits (MSU 1).
Can you use Sephadex G- 75 to separate alcohol dehydrogenase from bovine serum albumin? Why or why not?
Yes. The exclusion limits of the serum albumin would enable the separation (MSU 1).
How can you use the equation for Kd and the data in table 7.2 to calculate the exclusion limit for Sephadex G- 75?
Looking at the upper limits and the lower limits of the molecular weights, it can be seen that there are no molecular weights that are lower than 6,500 g/mole and there are no molecular weights that are above 48,000 g/mole. Any molecules that have a molecular weight higher than 48,000 g/ mole would be excluded by means of their particle size (MSU 1).
What characteristics of a column of Sephadex G- 75 determine if you can effectively separate cytochrome c from ?- lactalbumin?
The charac6teroitics of the columns would be to exclude molecules that have a smaller molecular weight than 12, 400 and higher than 15,500. The characteristics of the gel column would only enable molecules that have a weight between 12, 400 and 15, 500 (MSU 1).
Discussion
In the laboratory experiment, there had been many errors which were committed. The pipette accidentally fell into the gel. The protein became suspended on top. In addition, excess buffer had been added. The amount of buffer was corrected to twenty milliliters prior to starting to collect gel.
Furthermore, the separation of the protein in the mixture had not been complete. There had been a variety of inaccuracies in the sephadex gel. In the event that the column had not been repetitively replenished with buffer, the inaccuracy in the results could have been avoided. If the gel had formed cracks, the mystery substance and the elution would have migrated at a distinct rate. In addition, air bubbles could have deterred the gel formation. The pipette calibration may have affected the outcomes.
Conclusion
The aim of the experiment had been to detail the qualities of the mystery substance. The mystery substance was reviewed by means of the gel filtration and chromatography tests. The data revealed that the gel absorption spectrum and the light frequency wavelength to correlate with bovine albumin serum. The weight and the Kd values correlated this fact. In addition, the aim of the experiment had been to derive the antilog and to correlate it with the Kd values. This objective had been achieved in the fourth question of the Analysis of Results.
Works Cited
Bojesen. I. N. & E Bojesen. “Binding of arachidonate and oleate to bovine serum albumin.” J Lipid Res 35.5(1994): 770- 778.
Burden, David W. & Daniel B. Whitney. Biotechnology Proteins to PCR: A Course in Strategies and Lab Techniques. New York, NY: Springer Science and Business Media, 2012. Print.
MSU. ”Determining the molecular weight of amylase by gel filtration /size exclusion chromatography.” MSU, 2004. Web 2 November 2015. <https://www.msu.edu/course/lbs/chromatography04.pdf>
Steigel, Andre, Wallace W. Yau, Joseph L. Kirkland & Donald D. Bly. Modern Size- Exclusion Liquid Chromatography: Practice of Gel Permeation and Gel Filtration Chromatography. Second edition. Hoboken, NJ. John Wiley & Sons, Inc., 2003. Print.
Walsh, Gary. Proteins: Biochemistry and Biotechnology. Hoboken, NJ: John Wiley & Sons, Inc. 2002. Print.

Stuck with your Lab Report?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee