 All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Volume Error Propogation, Lab Report Example
Hire a Writer for Custom Lab Report
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

Introduction
Objective
The objective is to find the volume and calculate the uncertainty of each dimension of the cylinder, bullet, cup and the 6 balls and understand how the uncertainty of each dimension of the volume propagates the error of the volume of the bullet.
Experiment
Apparatus
- A bullet
- A cylinder
- A cup
- 6 balls
- Vernier Callipers
- Graphical analysis
Procedure
We took several measurements of the dimensions of the cylinders, 5 time using the Vernier calliper and recorded them.
We then took several measurements of the dimensions of the bullet and recorded them
We took several measurements of the dimensions of the cup and recorded them
We finally took several measurements of the dimensions of the 6 balls and recorded them.
We took the readings and input them into graphical analysis for each of the given objects
We calculated the mean and standard deviation of the readings using graphical analysis
We calculated the volume and uncertainty of the volume.
Experimental Data
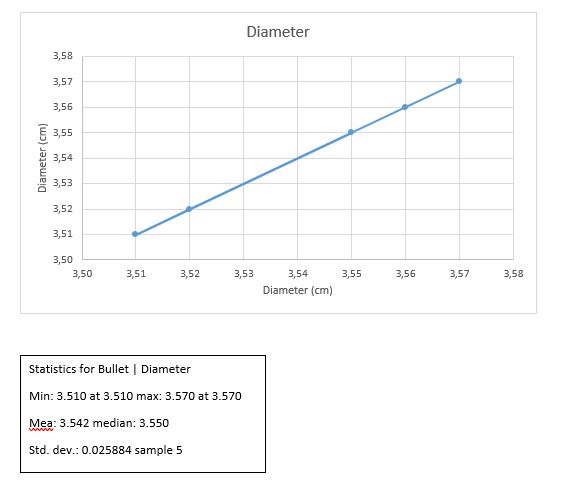
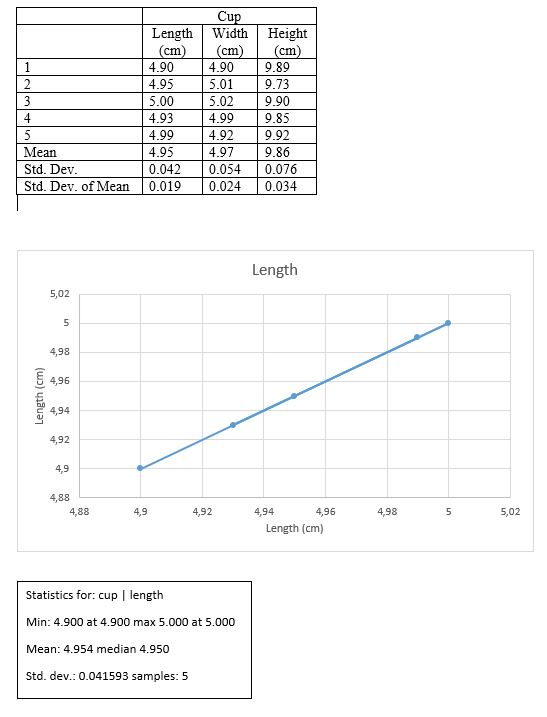
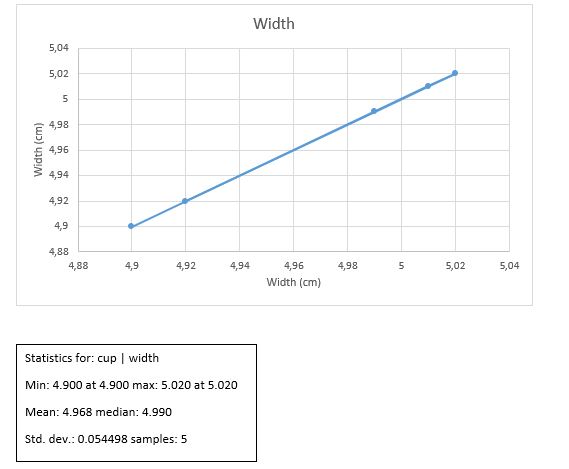
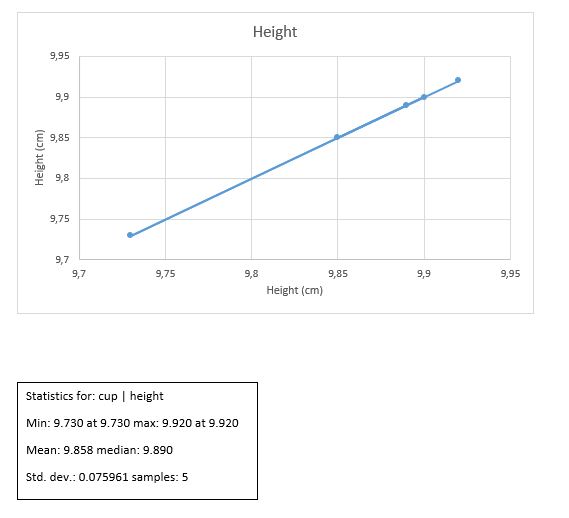
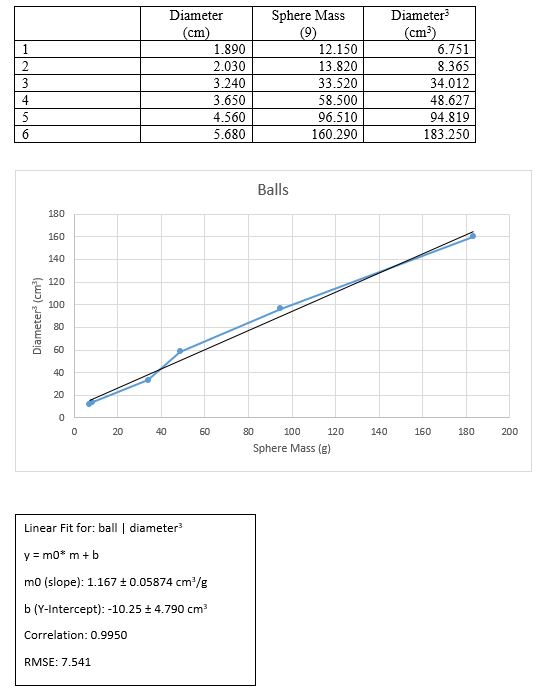
Results
Calculate the volume of the cup
H +/- ?H = 9.86 +/- 0.034 cm
L +/- ?L = 4.95 +/- 0.019 cm
W +/- ?W = 4.97 +/- 0.024 cm
V = H* L * W = 242.57 cm3
?V = [(?H * L * W)2+(2H * 2W *?L)2 + (H * 2L * ?W)2]1/2 = 4.48 cm3
V+/-?V=242.57 +/- 4.48cm3
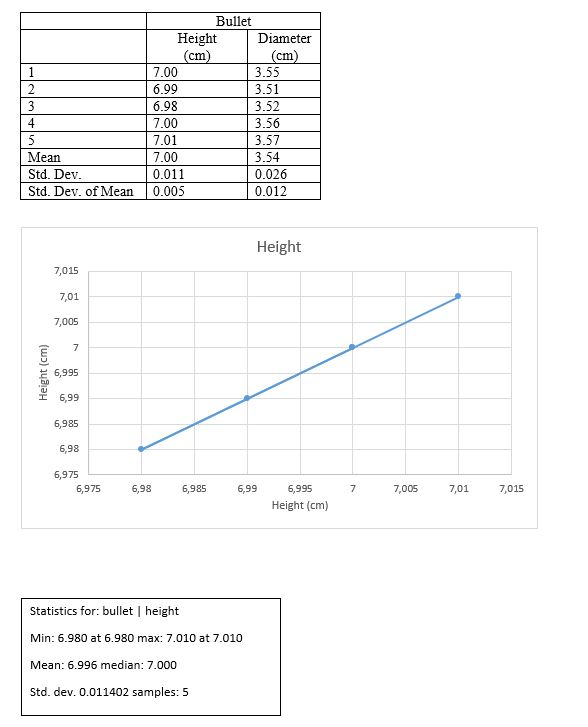
Calculate the volume for the bullet
D +/- ?D = 3.54 +/- 0.026
H +/- ?H = 7.00 +/- 0.011
V = Vcylinder + Vhemisphere = ¼ ?D2 (H-D/2) + 1/12 ?D3= ¼ (?D2H) – 1/24 (?D3)
= ¼ (? · 3.542 ·7.00) – 1/24 (? · 3.543)
= 68.90 – 5.81
= 63.09 cm3
?V = [(¼ · ? · D2 · ?H)2 + {(½ · ? · D · H – 1/8 · ? · D2) · ?D}2 ]1/2
= 0.94
V +/- ?V = 63.09 +/- 0.94 cm3
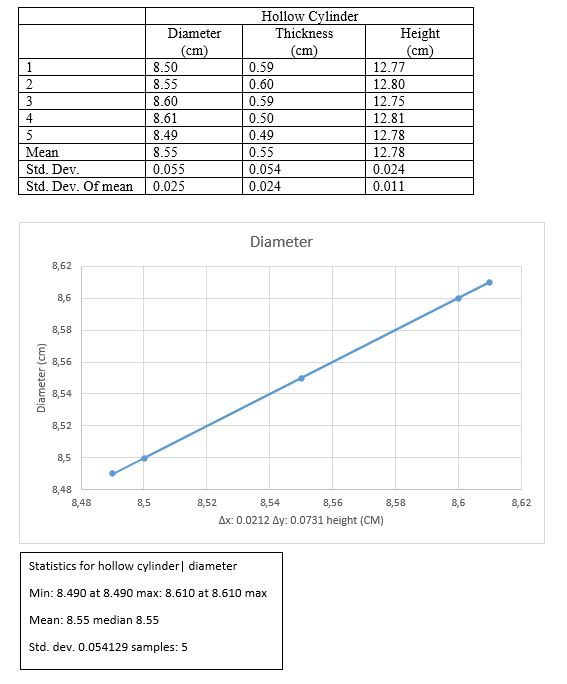
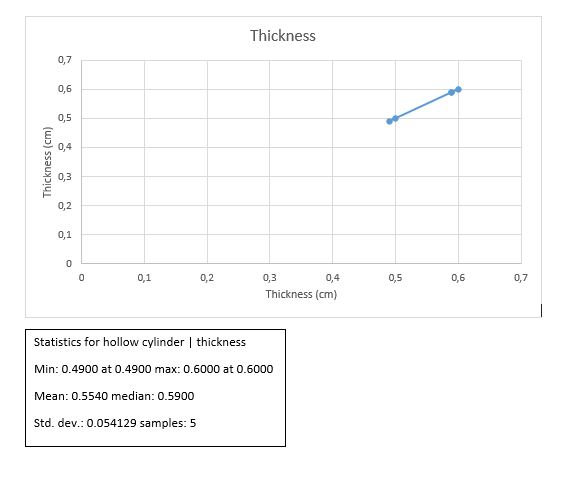
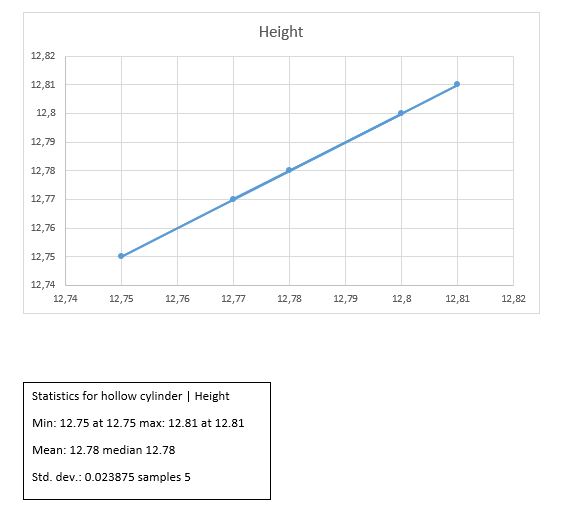
Hollow cylinder
H +/- ?H = 12.78 +/- 0.011cm
D +/- ?D = 8.55 +/- 0.025cm
T +/- ?T = 0.55 +/- 0.024cm
V=1/4 · ? (D2-T2)h = 730.72cm3
?V = ((1/4 · ? (D2-T2) ?h)2 + (0.5 · ? ·D · H · ?D)2 + (0.5 · ? · T · h · ?T)2)1/2
= 4.35 cm3
V +/- ?V = 730.72 +/- 4.35 cm3
Discussion
In this lab, the dimension of the bullet, cup, cylinder and the 6 balls were taken 5 times. The measurements of each of the dimensions of the objects were similar, although they did have a slight standard deviation. A suitable equation was configured for the calculation of the volume of the objects. A partial derivative was employed to help figure out the uncertainty of the volume
The actual value of the volume of the objects was found after plugging in the mean value and the standard deviation of the mean value of the volume of the bullet. The volume of the hollow cylinder can be derived from the subtraction of the volume of the inner cylinder from the volume of the outer cylinder. The volume of the whole bullet shape is found by adding the volume of the semi-sphere to the volume of the hollow cylinder. The results of the calculations make perfect sense.
Even though the value of the result of the experiment was not far off from the expected, the uncertainties associated with the bullet are high. The uncertainty associated with the height of the bullet was the highest and caused the large value in the overall uncertainty in the volume of the bullet. The most possible error in the experiment is due to our measurement of each dimension. The results of the experiment could be improved by conducting measurements more than five times. This leaves little room for error, reducing the uncertainty associated with the measurement of each dimension.
Conclusion
The volume and the uncertainty of volume was critically and carefully analysed. The error of the volume of the bullet is propagated by applying the partial derivative method. As the discussion above, the result of the volume and the uncertainty of the volume is reasonable. The possible errors are adequately covered in the discussion above.
In conclusion, the experiment met the objective of the volume lab.

Stuck with your Lab Report?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee

