 All papers examples
All papers examples
Disciplines

- MLA
- APA
- Master's
- Undergraduate
- High School
- PhD
- Harvard
- Biology
- Art
- Drama
- Movies
- Theatre
- Painting
- Music
- Architecture
- Dance
- Design
- History
- American History
- Asian History
- Literature
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- English
- Linguistics
- Law
- Criminal Justice
- Legal Issues
- Ethics
- Philosophy
- Religion
- Theology
- Anthropology
- Archaeology
- Economics
- Tourism
- Political Science
- World Affairs
- Psychology
- Sociology
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Anatomy
- Zoology
- Ecology
- Chemistry
- Pharmacology
- Earth science
- Geography
- Geology
- Astronomy
- Physics
- Agriculture
- Agricultural Studies
- Computer Science
- Internet
- IT Management
- Web Design
- Mathematics
- Business
- Accounting
- Finance
- Investments
- Logistics
- Trade
- Management
- Marketing
- Engineering and Technology
- Engineering
- Technology
- Aeronautics
- Aviation
- Medicine and Health
- Alternative Medicine
- Healthcare
- Nursing
- Nutrition
- Communications and Media
- Advertising
- Communication Strategies
- Journalism
- Public Relations
- Education
- Educational Theories
- Pedagogy
- Teacher's Career
- Statistics
- Chicago/Turabian
- Nature
- Company Analysis
- Sport
- Paintings
- E-commerce
- Holocaust
- Education Theories
- Fashion
- Shakespeare
- Canadian Studies
- Science
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
Paper Types

- Movie Review
- Essay
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article
- Article Critique
- Article Review
- Article Writing
- Assessment
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Case Study
- Coursework
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- Essay
- GCSE Coursework
- Grant Proposal
- Interview
- Lab Report
- Literature Review
- Marketing Plan
- Math Problem
- Movie Analysis
- Movie Review
- Multiple Choice Quiz
- Online Quiz
- Outline
- Personal Statement
- Poem
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Quiz
- Reaction Paper
- Research Paper
- Research Proposal
- Resume
- Speech
- Statistics problem
- SWOT analysis
- Term Paper
- Thesis Paper
- Accounting
- Advertising
- Aeronautics
- African-American Studies
- Agricultural Studies
- Agriculture
- Alternative Medicine
- American History
- American Literature
- Anatomy
- Anthropology
- Antique Literature
- APA
- Archaeology
- Architecture
- Art
- Asian History
- Asian Literature
- Astronomy
- Aviation
- Biology
- Business
- Canadian Studies
- Chemistry
- Chicago/Turabian
- Classic English Literature
- Communication Strategies
- Communications and Media
- Company Analysis
- Computer Science
- Creative Writing
- Criminal Justice
- Dance
- Design
- Drama
- E-commerce
- Earth science
- East European Studies
- Ecology
- Economics
- Education
- Education Theories
- Educational Theories
- Engineering
- Engineering and Technology
- English
- Ethics
- Family and Consumer Science
- Fashion
- Finance
- Food Safety
- Geography
- Geology
- Harvard
- Healthcare
- High School
- History
- Holocaust
- Internet
- Investments
- IT Management
- Journalism
- Latin-American Studies
- Law
- Legal Issues
- Linguistics
- Literature
- Logistics
- Management
- Marketing
- Master's
- Mathematics
- Medicine and Health
- MLA
- Movies
- Music
- Native-American Studies
- Natural Sciences
- Nature
- Nursing
- Nutrition
- Painting
- Paintings
- Pedagogy
- Pharmacology
- PhD
- Philosophy
- Physics
- Political Science
- Psychology
- Public Relations
- Relation of Global Warming and Extreme Weather Condition
- Religion
- Science
- Shakespeare
- Social Issues
- Social Work
- Sociology
- Sport
- Statistics
- Teacher's Career
- Technology
- Theatre
- Theology
- Tourism
- Trade
- Undergraduate
- Web Design
- West European Studies
- Women and Gender Studies
- World Affairs
- World Literature
- Zoology
Analysis of Ascorbic Acid by Redox Titration, Lab Report Example
Hire a Writer for Custom Lab Report
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.

Introduction
Ascorbic acid (C6H8O6), more commonly known as vitamin C, is a vital vitamin in mammals. In fact, the recommended daily intake of Vitamin C is 75-90 mg per day. Vitamin C is important because it functions as a cofactor in the synthesis of collagen, protein metabolism, iron absorption and the healing of wounds. (Lab Manual) Although vitamin C is an important substance, it is not produced by the body and must be ingested through diet and nutrition. Vitamin C is found in many foods; however, it is destroyed during cooking and therefore, fruits are the most reliable source of Vitamin C. In this lab, Vitamin C was determined in juice though oxidation-reduction reactions. The oxidation-reduction reaction using iodine was conducted. The solubility of iodine is triiodide. The triiodide oxidizes vitamin C to dehydroascorbic acid. The purpose of this experiment was to take unknown samples and perform the oxidation-reduction reactions in order to analyze the amount of ascorbic acid contained in the sample and compare it to the amount of ascorbic acid in juice.
Formulas and Equations
C6H8O6 + I3 – + H2O ® C6H6O6 + 3I- + 2H+ (2)
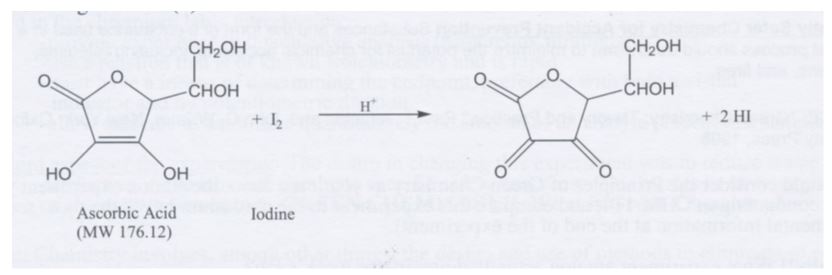
vitamin C dehydroascorbic acid
- Moles of Vitamin C oxidized= Equvilane point/ml
- Concentration of Vit C= Molecular Weight VitC *Molarity Iodine
- Grams Vit C used: Concentration Vit C x Volume Unknown
- Ka from pH of the half neutralization point (equation 4)
- Moles Vit C Oxidized= Molarity Iodine x Volume Iodine used
Procedure
- Unknown sample obtained.
- 150 ml iodine solution poured into 250 ml Erlenmeyer flask.
- One stir bar, 50 ml buret, 10 ml pipet obtained.
- 10ml of unknown transferred to 250ml beaker with 25ml distilled water.
- 10 drops of 2% starch added to solution. Starch used visual indicator used to follow the reaction.
- pH meter used to test pH of substance.
- Unknown sample titrated and total volume added versus mV readings recorded.
- Carefully watched for mV readings to change rapidly for the approaching of the endpoint.
- White sheet of paper placed underneath the sample to see endpoint.
- After the unknown samples analyzed, 25 ml of fruit juice titrated.
- Ascorbic acid concentration in the juice sample calculated.
- Waste flushed down the drain, due to “Green Chemistry” practice.
Data and Observations
Table 1. Unknown Titration Data
| Unknown Titration | #1 |
| Volume of Vit C unk solution | 10.00ml |
| ml of iodine used (visual/mV) | 17.43/270 (.065) |
| Moles of Vit C oxid. | .174 mol |
| Grams of Vit C oxid | .0176g |
| Conc of Vit C unknown (g/L) | 1.76g/L |
| Average Conc Vit C (visual/mV) | 27.3ml/305.1mV (.0894) |
Table 2. Orange Juice Titration Data
| Fruit Juice Titration | Orange Juice |
| Volume of Vit C unk solution | 25.0ml |
| ml of iodine used (visual/mV) | 17.38ml |
| Moles of Vit C oxid. | |
| Grams of Vit C oxid | |
| Conc of Vit C unknown (g/L) | |
| Average Conc Vit C (visual/mV) |
Table 3. Data from buret reading. Initial buret reading was 12.43 ml and initial mV was 270mV
| Buret Reading (ml) | Volt Meter Reading | Buret Reading | Volt Meter Reading |
| 18.3 | 264.2 | 28.5 | 245.0 |
| 18.5 | 265.8 | 28.72 | 245.0 |
| 19.0 | 265.3 | 28.98 | 246.6 |
| 19.5 | 266.4 | 29.22 | 247.9 |
| 20.0 | 265.5 | 29.38 | 282.9* Equivalence point |
| 20.5 | 265.0 | 29.77 | 286.0 |
| 21.0 | 263.6 | 30.00 | 295.0 |
| 21.5 | 267.3 | 30.5 | 360.0 |
| 22.0 | 261.4 | 30.7 | 362.4 |
| 22.5 | 259.7 | 30.9 | 369.2 |
| 23.0 | 256.8 | 31.1 | 373.9 |
| 23.2 | 254.6 | 31.3 | 379.3 |
| 23.4 | 253.8 | 31.4 | 388.0 |
| 23.6 | 252.9 | 31.6 | 391.4 |
| 23.8 | 251.8 | 31.8 | 391.7 |
| 24.0 | 250.9 | 32.0 | 393.1 |
| 24.2 | 250.2 | 32.2 | 394.0 |
| 24.6 | 249.4 | 32.4 | 394.8 |
| 24.8 | 248.2 | 32.6 | 395.3 |
| 25.0 | 247.6 | 32.8 | 396.3 |
| 25.5 | 247.5 | 33.0 | 397.0 |
| 26.0 | 247.2 | 33.2 | 397.3 |
| 26.5 | 246.6 | 33.4 | 398.0 |
| 27.0 | 246.0 | 33.6 | 398.8 |
| 27.5 | 245.6 | 33.8 | 398.9 |
| 28.0 | 245.3 | 34.0 | 399.5 |
| Avereage |
Table 4. Average Vitamin C concentration in Juices
| Type of Juice | Average Concentration of Vitamin C in Juice |
| Pink Grape Juice | 0.324 g/L |
| Orange Juice | 0.349 g/L |
| Apple Juice | 0.178 g/L |
| Pineapple Juice | 0.696 g/L |
Calculations
- Concentration of Vitamin C= Molecular Weigh Vit C * Molarity Iodine
- 1g/mol x 0.010 mol/L = 1.76 g/L
- Grams of Vit C oxidized= Concentration of Vitc C x Volume of unknown
- 76g/L x 10.00ml /1000ml=.0176g
- Moles of Vitamin C oxidized =.0100 mol/ml x 17.43ml= .174mol
Graphs
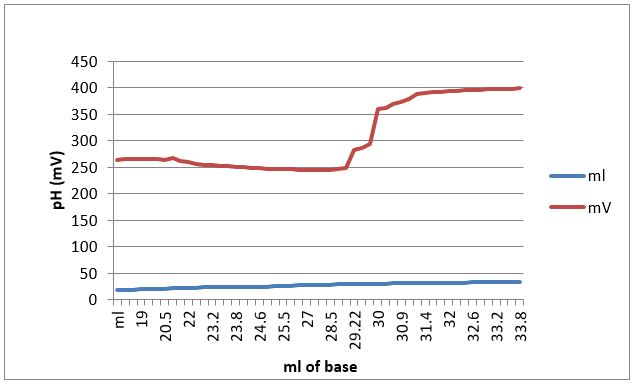
Figure 1. ph (mV) and ml of base used in the experiment for the unknown sample.
Discussion
This experiment analyzed an unknown substance using an oxidation reduction reaction in order to determine the concentration of ascorbic acid. In addition, the concentration of ascorbic in orange juice was determined. The experiment did go well. As seen in Figure 1, as the milliliters of base increase, so did the millivolts. This indicates that the ascorbic acid was oxidized. Our results did indicate that there was enough Vitamin C in our sample that is necessary for the recommended daily intake of 75 mg. Our results indicated that there was .0176 grams of Vitamin C (17.6mg). This is not enough for the daily intake. This illustrates how much food and drink that one would actually need in order to get the daily dosage that is recommended.
In this experiment, there were, however, some areas where there could have been human error. One, the volt meter might not have been calibrated properly and the results may have not been accurate. The meters were given to us already calibrated, therefore, we had to trust that the meters were properly calibrated. In addition, there was one person recording the readings while during the titrations. It is possible that some of the readings were not properly recorded by the lab partner.
This experiment was a “Green Chemistry” experiment. The point of Green Chemistry is that there are no harmful or carcinogenic toxic chemicals being released into the environment. The previous experiment used for this oxidation reduction lab used chromium. This chemical is toxic and is known as a carcinogen. Releasing this product into the environment can therefore have detrimental effects on living organisms. If chromium is used, it needs special waste disposal so that it is not released into the environment. This can lead to costly removal services for the school. The current experiment has left no need for a costly waste disposal and is not carcinogenic. For instance, the oxidized ascorbic acid made during this experiment is identical to the oxidized ascorbic acid that is excreted from humans on a daily basis and is not a toxic chemical. In addition, the iodine used in this laboratory served as a preventative measure for infections, thereby resulting in no waste problem as well. Furthermore, the final solution that was prepared during this experiment is very low on the acidity level. In fact, it is less than the acidity of the soda-pop, Coca-Cola.
Overall, this experiment illustrated how an oxidation reduction reaction occurred and it showed how chemistry can be “Green” even though you are working with chemicals.

Stuck with your Lab Report?
Get in touch with one of our experts for instant help!
Tags:

Time is precious
don’t waste it!
writing help!


Plagiarism-free
guarantee

Privacy
guarantee

Secure
checkout

Money back
guarantee

